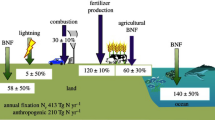
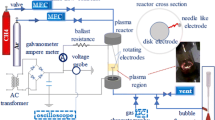
Abstract
In this paper, a three-level coupled rotating electrodes air plasma at atmospheric pressure is developed for evaluation of nitrogen fixation. Factors influencing the NOx production rate and energy cost, including airflow rate, the input H2O concentration, blade numbers at each rotating electrode and rotating speed, are examined. Air flow rates prove to have no effect on the rotational temperature of N2 337.1 nm and the emission intensities of N2+ and N2, but specific energy input (SEI) and species’ residence time can be shorter with higher air flow rates, resulting in lower NOx concentration and energy cost. The addition of H2O also has a positive effect on both NOx concentration and energy cost. Optical emission spectrum (OES) shows that air + H2O plasma has stronger 336 nm (NH) and 309 nm (OH) emission lines than air plasma, suggests NH and OH are the key species in NOx enhancement. The most energy efficient conditions are found at airflow rate of 15 l min−1, 12% H2O concentration, with 4 blades on each rotating speed. Under these conditions, the lowest energy cost is observed to be 165 GJ/tN.












Similar content being viewed by others
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of earth’s nitrogen cycle. Science 330:192
Chanway CP, Anand R, Yang H (2014) Advances in biology and ecology of nitrogen fixation
Bergman B, Sandh G, Lin S, Larsson J, Carpenter EJ (2013) Trichodesmium– a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol Rev 37:286–302
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276
Patil BS, Wang Q, Hessel V, Lang J (2015) Plasma N2-fixation: 1900–2014. Catal Today 256:49–66
Baltrusaitis J (2017) Sustainable ammonia production. ACS Sustain Chem Eng 5:9527
Cherkasov N, Ibhadon AO, Fitzpatrick P (2015) A review of the existing and alternative methods for greener nitrogen fixation. Chem Eng Process 90:24–33
Patil BS, Peeters FJJ, van Rooij GJ, Medrano JA, Gallucci F, Lang J, Wang Q, Hessel V (2018) Plasma assisted nitrogen oxide production from air: Using pulsed powered gliding arc reactor for a containerized plant. AlChE J 64:526–537
Gambarotta S, Scott J (2004) Multimetallic cooperative activation of N2. Angewandte Chemie Int Ed 43:5298–5308
Chen X, Li N, Kong Z, Ong W-J, Zhao X (2018) Photocatalytic fixation of nitrogen to ammonia: state-of-the-art advancements and future prospects. Mater Horizons 5:9–27
Wang W, Patil B, Heijkers S, Hessel V, Bogaerts A (2017) Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling. Chemsuschem 10:2110
Birkeland K (1906) On the oxidation of atmospheric nitrogen in electric arcs. Trans Faraday Soc 2:98–116
Hessel V, Cravotto G, Fitzpatrick P, Patil BS, Lang J, Bonrath W (2013) Industrial applications of plasma, microwave and ultrasound techniques: Nitrogen-fixation and hydrogenation reactions. Chem Eng Process: Process Intensif 71:19–30
Zhou D, Zhou R, Zhou R, Liu B, Zhang T, Xian Y, Cullen PJ, Lu X, Ostrikov K (2021) Sustainable ammonia production by non-thermal plasmas: Status, mechanisms, and opportunities. Chem Eng J 421:129544
Patil B (2017) Plasma (catalyst) assisted nitrogen fixation: reactor development for nitric oxide and ammonia production
Bogaerts A, Neyts EC (2018) plasma technology: an emerging technology for energy storage. ACS Energy Lett 3:1013–1027
Jardali F, Van Alphen S, Creel J, Ahmadi Eshtehardi H, Axelsson M, Ingels R, Snyders R, Bogaerts A (2021) NOx production in a rotating gliding arc plasma: potential avenue for sustainable nitrogen fixation. Green Chem 23:1748–1757
<Plasma Chemistry by Alexander Fridman (z-lib.org).pdf>
Petitpas G, Rollier JD, Darmon A, Gonzalez-Aguilar J, Metkemeijer R, Fulcheri L (2007) A comparative study of non-thermal plasma assisted reforming technologies. Int J Hydrogen Energy 32:2848–2867
Rusanov VD, Fridman AA, Sholin GV (1981) The physics of a chemically active plasma with nonequilibrium vibrational excitation of molecules. Soviet Phys Uspekhi 24:447–474
Mutel B, Dessaux O, Goudmand P (1984) Energy cost improvement of the nitrogen oxides synthesis in a low pressure plasma. Rev Phys Appl (Paris) 19:461–464
Patil BS, Cherkasov N, Lang J, Ibhadon AO, Hessel V, Wang Q (2016) Low temperature plasma-catalytic NOx synthesis in a packed DBD reactor: effect of support materials and supported active metal oxides. Appl Catal B 194:123–133
Malik MA, Jiang C, Heller R, Lane J, Hughes D, Schoenbach KH (2016) Ozone-free nitric oxide production using an atmospheric pressure surface discharge – A way to minimize nitrogen dioxide co-production. Chem Eng J 283:631–638
Patil BS, Rovira Palau J, Hessel V, Lang J, Wang Q (2016) Plasma nitrogen oxides synthesis in a milli-scale gliding arc reactor: investigating the electrical and process parameters. Plasma Chem Plasma Process 36:241–257
Janda M, Martišovitš V, Hensel K, Machala Z (2016) Generation of antimicrobial NOx by atmospheric air transient spark discharge. Plasma Chem Plasma Process 36:767–781
Hao X, Mattson AM, Edelblute CM, Malik MA, Heller LC, Kolb JF (2014) Nitric oxide generation with an air operated non-thermal plasma jet and associated microbial inactivation mechanisms. Plasma Processes Polym 11:1044–1056
Wang W, Patil B, Heijkers S, Hessel V, Bogaerts A (2017) Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling. Chemsuschem 10:2145–2157
Namihira T, Katsuki S, Hackam R, Akiyama H, Okamoto K (2002) Production of nitric oxide using a pulsed arc discharge. IEEE Trans Plasma Sci 30:1993–1998
Tsui YP, Cheh HY (1982) Quenching of air plasma effluents. Plasma Chem Plasma Process 2:387–398
Adamovich I, Rich J, Chernukho A, Zhdanok S (2000) 31st Plasmadynamics and Lasers Conference: American Institute of Aeronautics and Astronautics)
Rapakoulias D, Cavadias S, Amouroux J (1980) Processus catalytiques dans un réacteur à plasma hors d’équilibre II. Fixation de l’azote dans le système N2–O2. Rev Phys Appl (Paris) 15:1261–1265
Namihira T, Sakai S, Matsuda M, Wang D, Kiyan T, Akiyama H, Okamoto K, Toda K (2007) Temperature and nitric oxide generation in a pulsed arc discharge plasma. Plasma Sci Technol 9:747–751
Sakai S, Matsuda M, Wang D, Namihira T, Akiyama H, Okamoto K, Toda K (2009) Nitric oxide generator based on pulsed arc discharge. Acta Phys Pol, A 115:1104–1106
Korolev YD, Frants OB, Landl NV, Suslov AI (2012) Low-current plasmatron as a source of nitrogen oxide molecules. IEEE Trans Plasma Sci 40:2837–2842
Lu X, Keidar M, Laroussi M, Choi E, Szili EJ, Ostrikov K (2019) Transcutaneous plasma stress: from soft-matter models to living tissues. Mater Sci Eng R Rep 138:36–59
Malik MA (2016) Nitric oxide production by high voltage electrical discharges for medical uses: a review. Plasma Chem Plasma Process 36:737–766
Iwamoto M, Akiyama M, Aihara K, Deguchi T (2017) Ammonia synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu electrode catalysts in nonthermal atmospheric-pressure plasma of N2 and H2. ACS Catal 7:6924–6929
Shah J, Wang W, Bogaerts A, Carreon ML (2018) Ammonia synthesis by radio frequency plasma catalysis: revealing the underlying mechanisms. ACS Appl Energy Mater 1:4824–4839
Pei X, Gidon D, Graves DB (2018) Biologically active NOx production by nano-second pin-plate discharge in air. Clin Plasma Med 9:41
Pei X, Gidon D, Graves DB (2018) Propeller arc: design and basic characteristics. Plasma Sour Sci Technol 27:125007
Pei X, Gidon D, Yang Y-J, Xiong Z, Graves DB (2019) Reducing energy cost of NOx production in air plasmas. Chem Eng J 362:217–228
Rouwenhorst KHR, Jardali F, Bogaerts A, Lefferts L (2021) From the Birkeland-Eyde process towards energy-efficient plasma-based NOX synthesis: a techno-economic analysis. Energy Environ Sci 14:2520–2534
Ono R, Oda T (2001) OH radical measurement in a pulsed arc discharge plasma observed by a LIF method. IEEE Trans Ind Appl 37:709–714
Srivastava N, Wang C (2011) Effects of water addition on OH radical generation and plasma properties in an atmospheric argon microwave plasma jet. J Appl Phys 110:053304
Sun M, Wu Y, Li J, Wang NH, Wu J, Shang KF, Zhang JL (2005) Diagnosis of OH radical by optical emission spectroscopy in atmospheric pressure unsaturated humid air corona discharge and its implication to desulphurization of flue gas. Plasma Chem Plasma Process 25:31–40
Bruggeman P, Iza F, Guns P, Lauwers D, Kong MG, Gonzalvo YA, Leys C, Schram DC (2009) Electronic quenching of OH(A) by water in atmospheric pressure plasmas and its influence on the gas temperature determination by OH(A–X) emission. Plasma Sour Sci Technol 19:015016
Bruggeman P, Schram DC, Kong MG, Leys C (2009) Is the rotational temperature of OH(A–X) for discharges in and in contact with liquids a good diagnostic for determining the gas temperature? Plasma Process Polym 6:751–762
Bruggeman P, Schram D, González MÁ, Rego R, Kong MG, Leys C (2009) Characterization of a direct dc-excited discharge in water by optical emission spectroscopy. Plasma Sour Sci Technol 18:025017
Wang C, Wu W (2013) Simultaneous measurements of OH(A) and OH(X) radicals in microwave plasma jet-assisted combustion of methane/air mixtures around the lean-burn limit using optical emission spectroscopy and cavity ringdown spectroscopy. J Phys D: Appl Phys 46:464008
Park JY, Kostyuk PV, Han SB, Kim JS, Vu CN, Lee HW (2006) Study on optical emission analysis of AC air–water discharges under He, Ar and N2environments. J Phys D: Appl Phys 39:3805–3813
Liu F, Wang W, Zheng W, Wang Y (2006) Optical study of radicals (OH, O, H, N) in a needle-plate bi-directional pulsed corona discharge. Eur Phys J D - Atomic Mol, Opt Plasma Phys 38:515–522
Sarani A, Nikiforov AY, Leys C (2010) Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys Plasmas 17:063504
Zhang H, Zhu F, Li X, Cen K, Du C, Tu X (2016) Rotating Gliding Arc Assisted Water Splitting in Atmospheric Nitrogen. Plasma Chem Plasma Process 36:813–834
Hibert C, Gaurand I, Motret O, Pouvesle JM (1999) [OH(X)] measurements by resonant absorption spectroscopy in a pulsed dielectric barrier discharge. J Appl Phys 85:7070–7075
Eyde S (1912) Oxidation of atmospheric nitrogen and development of resulting industries in Norway. J Ind Eng Chem 4:771–774
Partridge WS, Parlin RB, Zwolinski BJ (1954) Fixation of nitrogen in a crossed discharge. Ind Eng Chem 46:1468–1471
Rahman M, Cooray V (2004) Erratum to NOx generation in laser-produced plasma in air as a function of dissipated energy: [Optics & Laser Technology 35 (2003) 543–546]. Optics & Laser Technology 36:85
Volynets AV, Lopaev DV, Rakhimova TV, Chukalovsky AA, Mankelevich YA, Popov NA, Zotovich AI, Rakhimov AT (2018) N2 dissociation and kinetics of N(4S) atoms in nitrogen DC glow discharge. J Phys D: Appl Phys 51:364002
Rehbein N, Cooray V (2001) NOx production in spark and corona discharges. J Electrostat 51–52:333–339
Pavlovich MJ, Ono T, Galleher C, Curtis B, Clark DS, Machala Z, Graves DB (2014) Air spark-like plasma source for antimicrobial NOx generation. J Phys D: Appl Phys 47:505202
Pei X, Gidon D, Graves DB (2019) Specific energy cost for nitrogen fixation as NO x using DC glow discharge in air. J Phys D: Appl Phys 53:044002
Kim T, Song S, Kim J, Iwasaki R (2010) Formation of NOx from air and N2/O2 mixtures using a nonthermal microwave plasma system. Jpn J Appl Phys 49:126201
Mutel B, Dessaux O, Goudmand P (1984) Energy cost improvement of the nitrogen oxides synthesis in a low pressure plasma. Revue de Physique Appliquée 19:461–464
Vervloessem E, Aghaei M, Jardali F, Hafezkhiabani N, Bogaerts A (2020) Plasma-based N2 fixation into NOx: insights from modeling toward optimum yields and energy costs in a gliding arc plasmatron. ACS Sustain Chem Eng 8:9711–9720
Lei X, Nie L, Xian Y, Lu X (2021) The effects of air flow on the nanosecond pulsed pin-to-pin discharge dynamics in atmosphere-pressure air. Phys Plasmas 28:053504
Pei X, Gidon D, Graves DB (2020) Specific energy cost for nitrogen fixation as NOx using DC glow discharge in air. J Phys D: Appl Phys 53:044002
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51625701 and 51977096)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, X., Cheng, H., Nie, L. et al. Nitrogen Fixation as NOx Enabled by a Three-Level Coupled Rotating Electrodes Air Plasma at Atmospheric Pressure. Plasma Chem Plasma Process 42, 211–227 (2022). https://doi.org/10.1007/s11090-021-10222-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10222-2