Abstract
Early clinical data indicate that some patients with castration-resistant prostate cancer may benefit from program death ligand-1 (PD-L1) inhibition, especially with enzalutamide. The IMbassador250 trial (no. NCT03016312) enrolled 759 men with metastatic castration-resistant prostate cancer whose disease progressed on abiraterone. The addition of atezolizumab to enzalutamide in an open-label randomized trial did not meet the primary endpoint of improved overall survival in unselected patients (stratified hazard ratio 1.12, 95% confidence interval (0.91, 1.37), P = 0.28), despite an acceptable safety profile. In archival tumor samples, prostate tumors showed comparatively low expression of key immune biomarkers. DNA damage-response alterations, phosphatase and tensin homolog status and PD-L1 expression levels were similar between hormone-sensitive and castration-resistant prostate cancers. In planned biomarker analysis, longer progression-free survival was seen with atezolizumab in patients with high PD-L1 IC2/3, CD8 expression and established immune gene signatures. Exploratory analysis linked progression-free survival in the atezolizumab arm with immune genes such as CXCL9 and TAP1, together with other potentially relevant biomarkers including phosphatase and tensin homolog alterations. Together these data indicate that the expected biology associated with response to immune checkpoint inhibitors is present in prostate cancer, albeit in fewer patients. Careful patient selection may be required for immune checkpoint inhibitors to identify subgroups of patients who may benefit from this treatment approach.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm. IMbassador250 raw data analyzed in this study have been submitted to the European Genome-Phenome Archive (EGA) with accession no. EGAS00001004852. Raw RNA-seq data from IMmotion150 and IMvigor210 have been submitted to EGA with accession no. EGAS00001004386.
References
Kirby, M., Hirst, C. & Crawford, E. D. Characterising the castration-resistant prostate cancer population: a systematic review. Int. J. Clin. Pract. 65, 1180–1192 (2011).
Logothetis, C. J. et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 13, 1210–1217 (2012).
Basch, E. et al. Development of the National Cancer Institute’s patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J. Natl Cancer Inst. 106, dju244 (2014).
Fizazi, K. et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 15, 1147–1156 (2014).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Antonarakis, E. S., Armstrong, A. J., Dehm, S. M. & Luo, J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. 19, 231–241 (2016).
Donahue, R. N. et al. Abstract 4901: short-course enzalutamide reveals immune activating properties in patients with biochemically recurrent prostate cancer. Cancer Res. 76, 4901 (2016).
Bishop, J. L. et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget 6, 234–242 (2015).
Graff, J. N. et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 7, 52810–52817 (2016).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014).
Beer, T. M. et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 35, 40–47 (2017).
TECENTRIQ (atezolizumab). Prescribing Information (Genentech, Inc., 2020).
TECENTRIQ (atezolizumab). Summary of Product Characteristics (Roche Registration Limited, 2020).
Petrylak, D. P. et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin. Cancer Res. 27, 3360–3369 (2021).
Hansen, A. R. et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann. Oncol. 29, 1807–1813 (2018).
Antonarakis, E. S. et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 38, 395–405 (2020).
Bou-Dargham, M. J., Sha, L., Sang, Q. A. & Zhang, J. Immune landscape of human prostate cancer: immune evasion mechanisms and biomarkers for personalized immunotherapy. BMC Cancer 20, 572 (2020).
Danaher, P. et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from The Cancer Genome Atlas (TCGA). J. Immunother. Cancer 6, 63 (2018).
Haffner, M. C. et al. Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am. J. Pathol. 188, 1478–1485 (2018).
Sharma, P. et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell 38, 489–499 (2020).
Graff, J. N. et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 8, e000642 (2020).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Feng, Q. & He, B. Androgen receptor signaling in the development of castration-resistant prostate cancer. Front Oncol. 9, 858 (2019).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Peng, W. et al. Loss of PTEN promotes resistance to t cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Jamaspishvili, T. et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 15, 222–234 (2018).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Mateo, J. et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J. Clin. Investig. 130, 1743–1751 (2020).
Powles, T. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391, 748–757 (2018).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Powles, T. et al. Atezolizumab (atezo) vs. chemotherapy (chemo) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC): immune biomarkers, tumor mutational burden (TMB), and clinical outcomes from the phase III IMvigor211 study. J. Clin. Oncol. 36, 409 (2018).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Kowanetz, M. et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc. Natl Acad. Sci. USA 115, E10119–E10126 (2018).
Farhood, B., Najafi, M. & Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol. 234, 8509–8521 (2019).
Fernandez-Poma, S. M. et al. Expansion of tumor-infiltrating CD8. Cancer Res. 77, 3672–3684 (2017).
Wu, Y. M. et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173, 1770–1782 (2018).
Sokol, E. S. et al. Pan-cancer analysis of CDK12 loss-of-function alterations and their association with the focal tandem-duplicator phenotype. Oncologist 24, 1526–1533 (2019).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Chan, T. A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 30, 44–56 (2019).
Konstantinopoulos, P. A. et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J. Clin. Oncol. 37, 2786–2794 (2019).
Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 5, 471–478 (2019).
Vidotto, T. et al. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 122, 1732–1743 (2020).
Ferraldeschi, R. et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur. Urol. 67, 795–802 (2015).
Powles, T. et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat. Med. 27, 793–801 (2021).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Fizazi, K. et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur. Urol. 78, 822–830 (2020).
Sharma, M., Yang, Z. & Miyamoto, H. Immunohistochemistry of immune checkpoint markers PD-1 and PD-L1 in prostate cancer. Medicine 98, e17257 (2019).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 34, 1402–1418 (2016).
Qi, Z. et al. Reliable gene expression profiling from small and hematoxylin and eosin-stained clinical formalin-fixed, paraffin-embedded specimens using the HTG EdgeSeq Platform. J. Mol. Diagn. 21, 796–807 (2019).
Chalmers, Z. R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Trabucco, S. E. et al. A novel next-generation sequencing approach to detecting microsatellite instability and pan-tumor characterization of 1000 microsatellite instability-high cases in 67,000 patient samples. J. Mol. Diagn. 21, 1053–1066 (2019).
Mateo, J. et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 21, 162–174 (2020).
Acknowledgements
The study was supported by F. Hoffmann-La Roche Ltd./Genentech, Inc., a member of the Roche Group. Disclosure forms, Methods and a data-sharing statement provided by the authors are available within the full text of this article at nature.com/nm. G.L.B. is partially funded by the Kaiser Permanente NCI National Community Oncology Research Program (grant no. SUG1CA189821-08). We thank the patients who participated in the trial and the clinical site investigators. Medical writing assistance for this manuscript was provided by P. Hong of Health Interactions, Inc. and was funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Contributions
Conceptualization was the responsibility of K.F., S.G., E.E.K., S.M., N.M., T.P., G.R., C.J.S. and P.W. Methodology was performed by A.D., S.M., N.M. and C.J.S. Validation was carried out by N.M. Formal analysis was conducted by A.D., P.W. and K.C.Y. Investigation was the responsibility of B.A., G.L.B., C.D., K.F., B.K., S.M., N.M., B.M., J.M.P., G.R., C.J.S. and P.J.W. Resources were acquired by B.A., G.L.B., K.F., N.M., J.M.P., G.R. and C.J.S. A.D., E.E.K., N.M., J.M.P., P.W. and K.C.Y. performed data curation. Writing, review and editing were done by B.A., G.L.B., A.D., J.F.D., C.D., K.F., S.G., E.E.K., B.K., S.M., N.M., B.M., J.M.P., T.P., G.R., D.R., C.J.S., P.W., P.J.W. and K.C.Y. Visualization was done by B.A., G.L.B., A.D., E.E.K., T.P., C.J.S. and K.C.Y. S.M., N.M., T.P., C.J.S. and P.W. carried out supervision. Project administration was done by S.M., N.M., T.P. and P.W. Funding acquisition was the responsibility of B.A. All authors were involved in further drafts of the manuscript and revised it critically for content. All authors gave final approval of the version to be published. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Competing interests
T.P. received honoraria from advisory/consultancy roles with AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono (EMD Serono), Astellas, Johnson & Johnson, Eisai and Roche; institutional research funding support from AstraZeneca, Roche, BMS, Exelixis, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono (EMD Serono), Astellas and Johnson & Johnson; and travel, accommodation and expenses support from Roche, Pfizer, MSD, AstraZeneca and Ipsen. S.G. received honoraria from Janssen; advisory/consultancy fees to the institution from Active Biotech, Astellas Pharma, Bayer, Bristol Myers Squibb, Clovis Oncology, CureVac, Ferring, Innocrin, Janssen, Menarini Silicon Biosystems and Novartis; advisory/consultancy fees from Advanced Accelerator Applications, Amgen, MaxiVax, Orion Pharma, Roche and Sanofi; and travel, accommodations and expenses from Nektar and ProteoMediX. S.G. also holds a patent involving a method for biomarker (no. WO 3752009138392 A1). K.C.Y. is an employee of Genentech and has stock ownership of Roche. E.E.K. is an employee of Genentech and has stock ownership of Roche, Clinuvel, Epizyme, Mannkind and Merck. D.R. received advisory/consultancy fees from AstraZeneca, Bayer, Genentech and Janssen; and institutional research funding support from AstraZeneca, Celgene, Ferring, Genentech/Roche, Janssen, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda and TRACON. N.M. received advisory/consultancy fees from Janssen, MSD, Chugai and Sanofi; and institutional research funding support from Janssen, MSD, Chugai, Astellas, Eli Lilly, Taiho and Pfizer. C.D. received advisory/consultancy fees from AstraZeneca/MedImmune, Bristol Myers Squibb, Compugen, Janssen Oncology, Merck, Pfizer, Pierre Fabre, Potenza Therapeutics, Roche/Genentech and Tizona Therapeutics; received institutional research funding support from Bristol Myers Squibb; owns stock and other ownership interests in Compugen, Harpoon Therapeutics, Kleo Pharmaceuticals and Tizona Therapeutics; and received travel, accommodations and expenses support from AACR, ASCO, Merck Sharp & Dohme, Pfizer and Roche/Genentech. C.D. also licenses patents through the institution to Bristol Myers Squibb and Potenza Therapeutics. K.F. received honoraria from Astellas Pharma, Janssen and Sanofi; received advisory/consultancy fees from Amgen, Astellas Pharma, AstraZeneca, Bayer, CureVac, ESSA Pharma, Janssen Oncology, Orion Pharma, Roche/Genentech and Sanofi; and travel, accommodations and expenses support from Amgen and Janssen. J.M.P. received advisory/consultancy fees from Astellas Pharma, Bristol Myers Squibb, Clovis Oncology, Janssen Oncology, Merck Sharp & Dohme, Roche/Genentech and VCN Biosciences; research funding support from AstraZeneca/MedImmune, Bristol Myers Squibb, Incyte, Janssen Oncology, Merck Sharp & Dohme and Pfizer/EMD Serono; and travel, accommodations and expenses support from Bristol Myers Squibb, Janssen Oncology and Roche. P.J.W. received honoraria from advisory/consultancy roles with AstraZeneca, Astellas, Bayer, Bristol Myers Squibb, Immunicom, Janssen, MSD, Merck, Novartis, Pfizer, Pierre Fabre, Roche and Sanofi. G.L.B. declares no conflict of interest. B.A. received grants from P. Herzen Oncology Research Institute during the conduct of the study; grants from AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Astellas, MSD, Eisai and Roche; personal fees from AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Astellas, MSD, Sanofi, Ferring, Ipsen, Eisai and Roche; and nonfinancial support from AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Astellas, MSD, Sanofi, Ferring and Roche. B.M. received advisory/consultancy fees to the institution from Amgen, Pfizer and Roche; advisory/consultancy fees from Astellas Pharma, AstraZeneca, Bayer, Janssen, Pfizer and Roche; research funding support from Bayer, Janssen and Roche; and travel, accommodations and expenses support from Janssen-Cilag and Roche. B.K. declares no conflict of interest. J.D. is an employee of Genentech and has stock ownership of Roche. G.R. is an employee of Roche and has stock ownership of Roche. A.D. is an employee of Roche and has stock ownership of Roche. S.M. is an employee of Genentech and has stock ownership of Roche. P.W. is an employee of Genentech and has stock ownership of Roche. C.J.S. received advisory/consultancy fees from Astellas, Pfizer, Janssen, Dendreon, Bayer, Genentech and Glaxo; and institutional research funding support from Astellas, Pfizer, Janssen, Dendreon, Bayer and Sanofi.
Additional information
Peer review information Nature Medicine thanks Lars Dyrskjøt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Javier Carmona was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
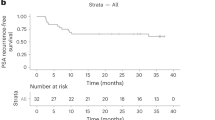
Extended Data Fig. 1 Forest plot of subgroup analysis.
(a) PFS and (b) rPFS in the intention-to-treat (ITT) population. P value and HR are from the unstratified Cox regression model. Prior local therapy included prior radical prostatectomy or radiotherapy. PD-L1–positive immune cells (IC) defined as: IC0, <1%; IC1/2/3, ≥1%; IC2/3, ≥5%.
Extended Data Fig. 2 Forest plot of known biomarkers in urothelial carcinoma, renal cell carcinoma and prostate cancer.
Biomarkers shown among urothelial carcinoma (IMvigor210), renal cell carcinoma (IMmotion150), and prostate cancer (IMbassador250) for PFS. DDR, DNA damage response; PD-L1, programmed death- ligand 1; PFS, progression-free survival; Teff, effector T cell, TMB, tumor mutational burden. Med refers to median PFS in months. HRs and CIs were calculated using Cox proportional hazards regression model, and P values were calculated using unstratified log-rank test without adjustment for multiplicity.
Extended Data Fig. 3 Exploratory analysis of DNA alterations in progression free survival.
PFS in the atezolizumab + enzalutamide vs enzalutamide treatment arms. 325 samples were included for analysis. DNA alteration biomarkers included Androgen Receptor (AR) amplification status, v-ets erythroblastosis virus E26 oncogene homolog (ERG) fusions, alterations of TP53, BRCA2, SPOP, CDK12 (at least a frameshift, nonsense or splice-site alteration) and ATM. In addition, frameshift mutation burden (FSB) was included. FSB was calculated by the number of frameshift mutations divided by length of genome examined. It was reported as the number of frameshift mutations per megabase (mut/Mb). The cutoff of FSB (8.7 mut/Mb) was previously established in prostate cancer. HRs and CIs were calculated using Cox proportional hazards regression model, and P values were calculated using log-rank test. MST refers to median survival time (PFS) in months.
Extended Data Fig. 4 Distribution of biomarkers and PD-L1 IC status.
Analysis of PTEN loss, Teff signature, DNA Damage Response (DDR) alterations and Androgen Receptor (AR) amplification status and PD-L1 IC status. IC0/1 were considered low IC whereas IC2/3 were considered high IC. Numbers on the bars indicate the number of patients being analysed.
Supplementary information
Supplementary Information
Supplementary Methods and Tables 1–7.
Rights and permissions
About this article
Cite this article
Powles, T., Yuen, K.C., Gillessen, S. et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med 28, 144–153 (2022). https://doi.org/10.1038/s41591-021-01600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01600-6
This article is cited by
-
Unlocking ferroptosis in prostate cancer — the road to novel therapies and imaging markers
Nature Reviews Urology (2024)
-
NIR-triggerable self-assembly multifunctional nanocarriers to enhance the tumor penetration and photothermal therapy efficiency for castration-resistant prostate cancer
Discover Nano (2023)
-
Immunogenomic profiles associated with response to life-prolonging agents in prostate cancer
British Journal of Cancer (2023)
-
Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration-resistant prostate cancer in an open-label Phase Ib study
British Journal of Cancer (2023)
-
Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study
Cancer Immunology, Immunotherapy (2023)