Abstract
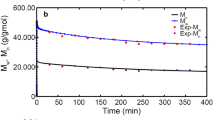
A hybrid approach that combines the method of moments and Monte Carlo simulation to predict the molecular weight distribution of low-density polyethylene for a continuous stirred tank reactor system is proposed. A ‘Block’, which is repeating reaction group, is introduced for the calculation cost-effective simulation. This model called the ‘block Kinetic Monte Carlo’ is ∼10 to 32 times faster than Neuhaus’s model. The model can be applied to any steady state system and provide a calculation cost reduction effect, where one reaction is much faster than others, for example, the propagation reaction. Furthermore, we performed a case study on the effects of the system temperature and initiator concentration on the MWD and reaction rate ratio. Based on the simulation results of 180 case studies, we determined a quantitative guideline for the appearance of shoulder, which is a function of the rate ratio of reactions to the propagation reaction.
Similar content being viewed by others
Abbreviations
- Ca :
-
concentration of molecule a [mol/L]
- Dn :
-
dead polymer chain of length n
- f:
-
effectiveness factor for initiator decomposition [dimensionless]
- Fsh :
-
shouldering measurement [dimensionless]
- k:
-
kinetic constant
- kb :
-
kinetic constant of beta scission reaction [s−1]
- kcp :
-
kinetic constant of chain transfer to polymer reaction [L(mol· s)−1]
- kcm :
-
kinetic constant of chain transfer to monomer reaction [L(mol· s)−1]
- kd :
-
kinetic constant of initiator decomposition reaction [s−1]
- kp :
-
kinetic constant of propagation reaction[L(mol·s)−1]
- kt :
-
kinetic constant of termination by combination reaction [L(mol·s)−1]
- Ln :
-
live polymer chain of length n
- Ln, sec :
-
secondary radical polymer chain of length n
- Llive :
-
chain length sampled from the live polymer chain length distribution
- Lmat :
-
the matrix where chain length data is stored
- M:
-
ethylene monomer
- N:
-
number of simulated taget chain
- Na :
-
number of molecules
- N AVO :
-
Avogadro number
- N B1 :
-
number of B1 blocks
- NB2 :
-
number of B2-1 and B2-2 blocks
- NB3 :
-
number of B3-1 and B3-2 blocks
- NB4 :
-
number of B4-1 and B4-2 blocks
- nrand :
-
random number between 0–1
- Nsamp :
-
total number of taget chains to be simulated
- Pp1, Pp2 :
-
Probabilities of propagation reaction
- Pt :
-
Probabilities of termination by combination reaction
- Pcp1, Pcp2 :
-
Probabilities of chain transfer to polymer reaction
- Pcm :
-
Probabilities of chain transfer to monomer reaction
- Pb1, Pb2 :
-
Probabilities of beta scission reaction
- r:
-
stochastic reaction rate
- Rj :
-
initiator decomposition reaction
- Rp1, Rp2 :
-
propagation reaction
- Rt :
-
termination by combination reaction
- Rcp1, Rcp2 :
-
chain transfer to polymer reaction
- Rcm :
-
chain transfer to monomer reaction
- Rbeta1, Rbeta2 :
-
beta scission reaction
- rp1, rp2 :
-
stochastic rate of propagation reaction [mol·s−1]
- rt :
-
stochastic rate of termination by combination reaction [mol·s−1]
- rcp1, rcp2 :
-
stochastic rate of chain transfer to polymer reaction [mol·s−1]
- rcm :
-
stochastic rate of chain transfer to monomer reaction [mol·s−1]
- rb1, rb2 :
-
stochastic rate of beta scission reaction [mol·s−1]
- T:
-
average residence time in CSTR reactor [sec]
- t:
-
current simulation time [sec]
- tsamp :
-
residence time of target chain [sec]
- V:
-
total volume of reactor system [L]
- v:
-
volumetric flow rate of input flow [L/s]
- [D]:
-
concentration of dead polymer [mol/L]
- [Dn]:
-
concentration of the dead polymer of length n [mol/L]
- [I]:
-
concentration of initiator [mol/L]
- [L]:
-
concentration of live polymer [mol/L]
- [Ln]:
-
concentration of the live polymer of length n [mol/L]
- [Lsec]:
-
concentration of secondary radical polymer [mol/L]
- [Lsec, n]:
-
concentration of the secondary radical polymer of length n [mol/L]
- [M]:
-
concentration of monomer [mol/L]
- λ k :
-
kth moment of live polymer chain
- λ sec, k :
-
kth moment of secondary radical polymer chain
- μ k :
-
kth moment of dead polymer chain
- CSTR:
-
continuous stirred-tank reactor
- CTA:
-
chain transfer agent
- HDPE:
-
high density polyethylene
- KMC:
-
kinetic monte carlo
- LDPE:
-
low density polyethylene
- MC:
-
monte carlo
- MI:
-
melt index
- MoM:
-
method of moment
- MWD:
-
molecular weight distribution
- PDI:
-
polydispersity index
- SMMA:
-
single macromolecule approach
- SVM:
-
support vector machine
References
F. A. Bovey, F. C. Schilling, F. L. McCrackin and H. L. Wagner, Macromolecules, 9, 76 (1976).
J. K. Beasley, J. Am. Chem. Soc., 75, 6123 (1953).
W. H. Ray, J. Macromol. Sci., Rev. Macromol. Chem., 8, 1 (1972).
T. J. Crowley and K. Y. Choi, Ind. Eng. Chem. Res., 36, 1419 (1997).
P. D. Iedema M. Wulkow and H. C. Hoefsloot, Macromolecules, 33, 7173 (2000).
S. H. Son, J. J. Han and J. M. Lee, Polymer, 126, 74 (2017).
B. M. Louie, G. M. Carratt and D. S. Soong, J. Appl. Polym. Sci., 30, 3985 (1985).
A. G. Mikos, C. G. Takoudis and N. A. Peppas, Macromolecules, 19, 2174 (1986).
S. Zhu and A. E. Hamielec, Macromolecules, 22, 3093 (1989).
S. Zhu and A. E. Hamielec, J. Polym. Sci. Part B: Polym. Phys., 32, 929 (1994).
S. Shin, S. Choi, J. Na, I. Jung, M.-K. Kim, M.-J. Park and W.B. Lee, Chem. Eng. J., 131829, https://doi.org/10.1016/j.cej.2021.131829.
G. J. Wells and W. H. Ray, Macromol. Mater. Eng., 290, 319 (2005).
P. Pladis and C. A. Kiparissides, Chem. Eng. Sci., 53, 3315 (1998).
R. A. Hutchinson, Macromol. Theory Simul., 10, 144 (2001).
S. X. Zhang and W. H. Ray, AIChE J., 43, 1265 (1997).
H. Tobita, J. Polym. Sci., Part B: Polym. Phys., 39, 391 (2001).
N. Yaghini and P. D. Iedema, Chem. Eng. Sci., 116, 144 (2014).
D. Meimaroglou, P. Pladis, A. Baltsas and C. Kiparissides, Chem. Eng. Sci., 66, 1685 (2011).
D. M. Kim, M. Busch, H. C. Hoefsloot and P. D. Iedema, Chem. Eng. Sci., 59, 699 (2004).
C. Kiparissides, A. Krallis, D. Meimaroglou, P. Pladis and A. Baltsas, Chem. Eng. Technol., 33, 1754 (2010).
P. D. Iedema and H. C. Hoefsloot, Macromol. Theory Simul., 10, 855 (2001).
D. M. Kim and P. D. Iedema, Chem. Eng. Sci., 59, 2039 (2004).
D. M. Kim and P. D. Iedema, Chem. Eng. Sci., 63, 2035 (2008).
N. Yaghini and P. D. Iedema, Chem. Eng. Sci., 130, 310 (2015).
D. T. Gillespie, J. Phys. Chem., 81, 2340 (1977).
H. Tobita, Macromol. Theory Simul., 5, 129 (1996).
H. Tobita, Macromol. React. Eng., 7, 181 (2013).
H. Tobita, Macromol. Theory Simul., 23, 182 (2014).
M. Rogošić, H. J. Mencer and Z. Gomzi, Eur. Polym. J., 32, 1337 (1996).
E. Neuhaus, T. Herrmann, I. Vittorias, D. Lilge, G. Mannebach, A. Gonioukh and M. Busch, Macromol. Theory Simul., 23, 415 (2014).
C. Schütte and M. Wulkow, Macromol. React. Eng., 4, 562 (2010).
D. Eckes and M. Busch, Macromol. Symp., 360, 23 (2016).
D. Meimaroglou and C. A. Kiparissides, Macromolecules, 43, 5820 (2010).
P. Feucht, B. Tilger and G. Luft, Chem. Eng. Sci., 40, 1935 (1985).
H. Tobita, Processes, 3, 731 (2015).
I. L. Chien, T. W. Kan and B. S. Chen, Comput. Chem. Eng., 31, 233 (2007).
A. M. Kotliar, J. Polym. Sci., Part A: Gen. Pap., 2, 4303 (1964).
A. Krallis, P. Pladis and C. Kiparissides, Macromol. Theory Simul., 16, 593 (2007).
N. H. Kolhapure and R. O. Fox, Chem. Eng. Sci., 54, 3233 (1999).
L. Marini and C. Georgakis, Chem. Eng. Commun., 30, 361 (1984).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C1005503).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S., Lee, Y., Park, S. et al. Molecular weight distribution modeling of LDPE in a continuous stirred-tank reactor using coupled deterministic and stochastic approach. Korean J. Chem. Eng. 39, 798–810 (2022). https://doi.org/10.1007/s11814-021-0943-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0943-9