Abstract
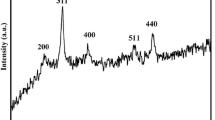
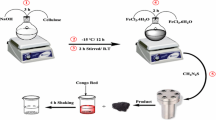
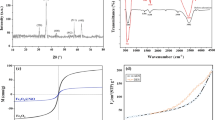
We report here a simple approach for synthesis of carbon coated magnetite (C@MFe2O4, M=Co, Ni, Fe) with shell@core nanostructured composites that we used as magnetic-nanosorbents for direct yellow (DYG) and moderacid red (RS) as pollutant textile dyes removal via an adsorption process. The synthesized C@MFe2O4 was characterized by TEM, SEM, EDX, XRD, FT-IR and VSM techniques. TEM results indicated that C@MFe2O4 nanocomposites have 20–30 nm of MFe2O4 nanoparticle core and 2–3 nm in thickness of the amorphous carbon shell. The synthesized C@MFe2O4 nanocomposites have the zero point charge (pHZPC) at 5.5, which suggests that DYG and RS, anionic dyes can be adsorbed onto the C@MFe2O4 nanosorbents in the acidic medium. Adsorption of DYG and RS onto magnetic nanosorbents was optimized and adsorption thermodynamic parameters were evaluated, clearly indicating that the adsorption of RS onto synthesized magnetic-nanosorbents was facile more than that DYG. The adsorption isotherm data showed that the adsorption processes of DYG and RS onto Fe3O4 or C@MFe2O4 nanosorbents are more suitable for the Langmuir model than Freundlich model. The maximum adsorption capacity (qmax) of DYG dye onto Fe3O4, C@Fe3O4 and C@CoFe2O4 adsorbents was 14.641, 36.232 and 7.85 mg g−1, respectively; meanwhile, these values were 41.152, 61.728 and 39.683 mg g−1 for RS dye. These obtained data indicate that the developed Fe3O4, C@Fe3O4 and C@CoFe2O4 nanoparticles can be used as recoverable and recyclable adsorbents for not only organic pigments adsorption but also for heavy metal ion removal or protein extraction as well.
Similar content being viewed by others
References
S. Abedi and F. Nekouei, E-J. Chem., 8, 1588 (2011).
G. Revathi, S. Ramalingam, P. Subramaniam and A. Ganapathi, E-J. Chem., 8, 1536 (2011).
A. E.-A. A. Said, A. A. M. Aly, M. M. A. El-Wahab, S. A. E.-F. Soliman, A. A. A. El-Hafez, V. Helmey and M. N. Goda, Energy Environ. Eng., 1, 10 (2013).
N. T.-T. Hoang, A. T.-K. Tran, M.-H. Hoang, T. T. H. Nguyen and X.-T. Bui, Environ. Technol. Innov., 21, 101255 (2020).
V. L. Silva, G. Dilarri, C. R. Mendes, R. B. Lovaglio, A. R. Gonçalves, R. N. Montagnolli and J. Contiero, J. Mol. Liq., 321, 114753 (2021).
H. V. Tran, L. T. Hoang and C. D. Huynh, Chem. Phys., 535, 110793 (2020).
H. V. Tran, T. L. Tran, T. D. Le, T. D. Le, H. M. T. Nguyen and L. T. Dang, Mater. Res. Express, 6, 025018 (2018).
H. V. Tran, L. T. Bui, T. T. Dinh, D. H. Le, C. D. Huynh and A. X. Trinh, Mater. Res. Express, 4, 035701 (2017).
P. P. Hankare, R. P. Patil, A. V. Jadhav, K. M. Garadkar and R. Sasikala, Appl. Catal. B: Environ., 107, 333 (2011).
M. C. Ceballos-Chuc, C. M. Ramos-Castillo, J. J. Alvarado-Gil, G. Oskam and G. Rodríguez-Gattorno, J. Phys. Chem. C, 122, 19921 (2018).
Z. A. Al Othman, M. A. Habila, R. Ali, A. A. Ghafar and M. S. Eldin Hassouna, Arabian J. Chem., 7, 1148 (2014).
A. Kumar and H. M. Jena, J. Clean. Prod., 137, 1246 (2016).
E. I. El-Shafey, S. N. F. Ali, S. Al-Busafi and H. A. J. Al-Lawati, J. Environ. Chem. Eng., 4, 2713 (2016).
C. A. P. Almeida, N. A. Debacher, A. J. Downs, L. Cottet and C. A. D. Mello, J. Colloid Interface Sci., 332, 46 (2009).
L. Wang, J. Zhang and A. Wang, Colloids Surf. A: Physicochem. Eng. Asp., 322, 47 (2008).
D. Dutta, D. Thakur and D. Bahadur, Chem. Eng. J., 281, 482 (2015).
H. Mittal, A. Maity and S. S. Ray, Chem. Eng. J., 279, 166 (2015).
G. M. K. Tolba, A. M. Bastaweesy, E. A. Ashour, W. Abdelmoez, K. A. Khalil and N. A. M. Barakat, Arabian J. Chem., 9, 287 (2016).
D. M. EL-Mekkawi, F. A. Ibrahim and M. M. Selim, J. Environ. Chem. Eng., 4, 1417 (2016).
T. S. Jamil, H. H. A. Ghafar, H. S. Ibrahim and I. H. A. El-Maksoud, Solid State Sci., 13, 1844 (2011).
A. K. Hammed, N. Dewayanto, D. Du, M. H. A. Rahim and M. R. Nordin, J. Environ. Chem. Eng., 4, 2607 (2016).
Z. Shen, X. Fan, D. Hou, F. Jin, D. O’Connor, D. C. W. Tsang, Y. S. Ok and D. S. Alessi, Chemosphere, 233, 149 (2019).
S. S. Yang, Y. Chen, Y. Zhang, H. M. Zhou, X. Y. Ji, L. He, D. F. Xing, N. Q. Ren, S. H. Ho and W. M. Wu, Environ. Pollut., 252, 1142 (2019).
N. Amin, A. Hussain, S. Alamzeb and S. Begum, Food Chem., 136, 1515 (2013).
H. Treviño-Cordero, L. G. Juárez-Aguilar, D. I. Mendoza-Castillo, V. Hernández-Montoya, A. Bonilla-Petriciolet and M. A. Montes-Morán, Ind. Crops Products, 42, 315 (2013).
H. Kominko, K. Gorazda and Z. Wzorek, J. Environ. Manage., 248, 109283 (2019).
Z. M. Xu, Z. Wang, Q. Gao, L.-L. Wang, L.-L. Chen, Q.-G. Li, J.-J. Jiang, H.-J. Ye, D.-S. Wang and P. Yang, J. Environ. Manage., 244, 453 (2019).
A. Saeed, M. Iqbal and M. W. Akhtar, J. Hazard. Mater., 117, 65 (2005).
K. Page, M. J. Harbottle, P. J. Cleall and T. R. Hutchings, Sci. Total Environ., 487, 260 (2014).
N. Gupta, A. K. Kushwaha and M. C. Chattopadhyaya, Arabian J. Chem., 9, S707 (2016).
W. Hassan, U. Farooq, M. Ahmad, M. Athar and M. A. Khan, Arabian J. Chem., 10, S1512 (2017).
A. J. B. Leite, E. C. Lima, G. S. dos Reis, P. S. Thue, C. Saucier, F. S. Rodembusch, S. L. P. Dias, C. S. Umpierres and G. L. Dotto, J. Environ. Chem. Eng., 5, 4307 (2017).
R. R. Schio, B. C. da Rosa, J. O. Gonçalves, L. A. A. Pinto, E. S. Mallmann and G. L. Dotto, Int. J. Biol. Macromol., 121, 373 (2019).
M. Atrous, L. Sellaoui, M. Bouzid, E. C. Lima, P. S. Thue, A. Bonilla-Petriciolet and A. B. Lamine, J. Mol. Liq., 294, 111610 (2019).
M. Kosmulski, J. Colloid Interface Sci., 275, 214 (2004).
M. Kosmulski, J. Colloid Interface Sci., 298, 730 (2006).
A. Ebrahimian Pirbazari, E. Saberikhah and S. S. H. Kozani, Water Resour. Ind., 7–8, 23 (2014).
L. Wang, J. Li, Y. Wang, L. Zhao and Q. Jiang, Chem. Eng. J., 181–182, 72 (2012).
A. Millan, A. Urtizberea, N. J. O. Silva, F. Palacio, V. S. Amaral, E. Snoeck and V. Serin, J. Magn. Magn. Mater., 312, L5 (2007).
M. Stoia, C. Păcurariu, R. Istratie and D. Nižńanský, J. Therm. Anal. Calorim., 121, 989 (2015).
X. Bao, Z. Qiang, J.-H. Chang, W. Ben and J. Qu, J. Environ. Sci., 26, 962 (2014).
W. Zhang, L. Y. Zhang, X. J. Zhao and Z. Zhou, J. Mol. Liq., 222, 995 (2016).
G. Z. Kyzas, E. A. Deliyanni and K. A. Matis, J. Chem. Technol. Biotechnol., 89, 196 (2014).
L. Slavov, M. V. Abrashev, T. Merodiiska, C. Gelev, R. E. Vandenberghe, I. Markova-Deneva and I. Nedkov, J. Magn. Magn. Mater., 322, 1904 (2010).
P. C. Panta and C. P. Bergmann, J. Material. Sci. Eng., 5, 1 (2015).
F. Sotomayor, K. A. Cychosz and M. Thommes, Accounts Mater. Surf. Res., 3, 34 (2018).
Acknowledgement
This work was financially supported by the Ministry of Education and Training of Vietnam under project code B2020-SPH-02.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supporting Information
Additional information as noted in the text. This information is available via the Internet at http://www.springer.com/chemistry/journal/11814.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Tran, H.V., Nguyen, H.V., Vu, D.V. et al. Carbon coated MFe2O4 (M=Fe, Co, Ni) magnetite nanoparticles: A smart adsorbent for direct yellow and moderacid red dyes. Korean J. Chem. Eng. 39, 431–439 (2022). https://doi.org/10.1007/s11814-021-0905-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0905-2