Abstract
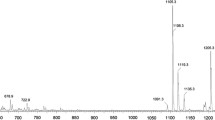
The results of an analysis for ginsenosides in the Multiphytoadaptogene (MPA) phytoadaptogene composition with antitumor properties by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) are presented. Ginsenosides are identified with the use of commercial standards of ginsenosides and some literature data. Ginsenosides Rb1, Rb2, Rc, Rd, Rg1, Rg2, Re, Rf, and Ro are revealed in MPA. The results could be used for the standardization and quality control of phytocomplexes containing triterpenoid glycosides.




Similar content being viewed by others
REFERENCES
Bocharova, O.A., Karpova, R.V., Bocharov, E.V., Vershinskaya, A.A., Baryshnikova, M.A., Kazeev, I.V., Kucheryanu, V.G., Kiselevsky, M.V., et al., Phytoadaptogens in biotherapy of tumors and geriatrics (Part 1), Ross. Bioterapevticheskii Zh., 2020, vol. 19, no. 2, p. 13.
Bocharova, O.A., Karpova, R.V., Bocharov, E.V., Vershinskaya, A.A., Baryshnikova, M.A., Kazeev, I.V., Kucheryanu, V.G., and Kiselevsky, M.V., Phytoadaptogens in biotherapy of tumors and geriatrics (Part 2), Ross. Bioterapevticheskii Zh., 2020, vol. 19, no. 3, p. 12.
Liu, T., Zhao, L., Hou, H., et al., Ginsenoside 20(S)-Rg3 suppresses ovarian cancer migration via hypoxia-inducible factor 1 alpha and nuclear factor-kappa B signals, Tumor Biol., 2017, vol. 39, no. 5. https://doi.org/10.1177/1010428317692225
Vinh, L.B., Park, J.U., Duy, L.X., et al., Ginsenosides from Korean red ginseng modulate T cell function via the regulation of NF-AT-mediated IL-2 production, Food Sci. Biotechnol., 2019, vol. 28, no. 1, p. 237. https://doi.org/10.1007/s10068-018-0428-8
Meng, Q., Pan, J., Liu, Y., et al., Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity, Exp. Ther. Med., 2018, vol. 15, no. 2, p. 1694. https://doi.org/10.3892/etm.2017.5568
Le, H.T., Nguyen, H.T., Min, H.Y., Hyun, S.Y., Kwon, S., Lee, Y., Van Le, T.H., Lee, J., Park, J.H., and Lee, H.-Y., Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells, Cancer Lett. (N. Y., NY, U. S.), 2018, vol. 412, p. 297. https://doi.org/10.1016/j.canlet.2017.10.013
Bocharova, O.A., Karpova, R.V., Bocharov, E.V., Vershinskaya, A.A., Baryshnikova, M.A., Kazeev, I.V., Kucheryanu, V.G., Kiselevsky, M.V., and Matveev, V.B., Research of new phytoadaptogens and possibilities of herbal formulas application, Ross. Bioterapevticheskii Zh., 2020, vol. 19, no. 4, p. 35.
Sheychenko, V.I., Bocharova, O.A., Sheychenko, O.P., Bocharov, E.V., and Bykov, V.A., Analytical capabilities of the NMR method for determining the components of Phitomix-40, Ind. Lab., Diagn. Mater., 2006, vol. 72, no. 8, p. 15.
Sheychenko, O.P., Bocharova, O.A., Sheychenko, V.I., Tolkachev, O.N., Bocharov, E.V., Karpova, R.V., and Bykov, V.A., Possibility of using electronic absorption spectra for standardization of the multicomponent preparation “Phytomix-40", Probl. Biol., Med. Pharm. Chem., 2007, vol. 5, no. 2, p. 20.
Sheychenko, O.P., Bocharova, O.A., Krapivkin, B.A., Uyutova, E.V., Karpova, R.V., Kazeev, I.V., Bocharov, E.V., and Bykov, V.A., Investigation of complex phytoadaptogen by HPLC, Probl. Biol., Med. Pharm. Chem., 2012, vol. 10, p. 52.
Kazeev, I.V., Bocharova, O.A., Shevchenko, V.E., Karpova, R.V., Bocharov, E.V., Uyutova, E.V., Sheychenko, O.P., Kucheryanu, V.G., and Baryshnikova, M.A., Tandem mass spectrometry in the technology of determining aralosides of phytoadaptogene compositions, Theor. Found. Chem. Eng., 2020, vol. 54, no. 6, pp. 1242–1246. https://doi.org/10.1134/S0040579520050334
Mancuso, C. and Santangelo, R., Panax ginseng and Panax quinquefolius: From pharmacology to toxicology, Food Chem. Toxicol., 2017, vol. 107, part A, pp. 362–372. https://doi.org/10.1016/j.fct.2017.07.019
Taira, S., Uematsu, K., Kaneko, D., and Katano, H., Mass spectrometry imaging: Applications to food science, Anal. Sci., 2014, vol. 30, no. 2, p. 197.
Liu, Z., Li, Y., Li, X., Ruan, C.-C., Wang, L.-J., and Sun, G.-Z., The effects of dynamic changes of malonyl ginsenosides on evaluation and quality control of Panax ginseng C.A. Meyer, J. Pharm. Biomed. Anal., 2012, vols. 64–65, p. 56.
Morinaga, O., Uto, T., Yuan, C.S., et al., Evaluation of a new eastern blotting technique for the analysis of ginsenoside Re in American ginseng berry pulp extracts, Fitoterapia, 2010, vol. 81, no. 4, p. 284.
Zhao, Q., Zheng, X., Jiang, J., et al., Determination of ginsenoside Rg3 in human plasma and urine by high performance liquid chromatography-tandem mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, vol. 878, no. 24, p. 2266.
Lee, S.M., Bae, B.-S., Park, H.-W., Ahn, N.-G., et al., Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition, J. Ginseng Res., 2015, vol. 39, no. 4, p. 384. https://doi.org/10.1016/j.jgr.2015.04.009
Lv, W.J., Liu, C., Li, Y.F., Chen, W.Q., et al., Systems pharmacology and microbiome dissection of Shen Ling Bai Zhu San reveal multiscale treatment strategy for IBD, Oxid. Med. Cell. Longevity, 2019, vol. 2019, article no. 8194804. https://doi.org/10.1155/2019/8194804
Shi, Z.Y., Zeng, J.Z., and Wong, A.S.T., Chemical structures and pharmacological profiles of ginseng saponins, Molecules, 2019, vol. 24, no. 13, p. 2443. https://doi.org/10.3390/molecules24132443
Nguyen, N.H. and Nguyen, C.T., Pharmacological effects of ginseng on infectious diseases, Inflammopharmacology, 2019, vol. 27, no. 5, p. 871. https://doi.org/10.1007/s10787-019-00630-4
Bocharova, O.A., Bocharov, E.V., Karpova, R.V., Il’enko, V.A., Kazeev, I.V., and Baryshnikov, A.Yu., Integrins LFA-1 and Mac-1 and cytokines IL-6 and IL-10 in high-cancer mice under influence of phytoadaptogen, Bull. Exp. Biol. Med., 2014, vol. 157, no. 2, pp. 258–260. https://doi.org/10.1007/s10517-014-2539-4
Kim, D.H., Kim, D.W., Jung, B.H., Lee, J.H., Lee, H., Hwang, G.S., Kang, K.S., and Lee, J.W., Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress in neuronal cell, J. Ginseng Res., 2019, vol. 43, no. 2, p. 326. https://doi.org/10.1016/j.jgr.2018.12.002
Chen, J., Li, M., Qu, D., and Sun, Y., Neuroprotective effects of red ginseng saponins in scopolamine-treated rats and activity screening based on pharmacokinetics, Molecules, 2019, vol. 24, no. 11, p. 2136. https://doi.org/10.3390/molecules24112136
Alessio, N., Capasso, S., Di Bernardo, G., Cappabianca, S., Casale, F., Calarco, A., Cipollaro, M., Peluso, G., and Galderisi, U., Mesenchymal stromal cells having inactivated RB1 survive following low irradiation and accumulate damaged DNA: Hints for side effects following radiotherapy, Cell Cycle, 2017, vol. 16, no. 3, p. 251. https://doi.org/10.1080/15384101.2016.1175798
Tian, Y.Z., Liu, Y.P., Tian, S.C., Ge, S.Y., Wu, Y.J., and Zhang, B.L., Antitumor activity of ginsenoside Rd in gastric cancer via up-regulation of Caspase-3 and Caspase-9, Pharmazie, 2020, vol. 75, no. 4, p. 147. https://doi.org/10.1691/ph.2020.9931
Bocharova, O.A., Davydov, M.I., Klimenkov, A.A., Baryshnikov, A.Y., Karpova, R.V., Chulkova, S.V., Gorozhanskaya, E.G., and Ilyenko, V.A., Prospects of using phytoadaptogen in the treatment of diffuse stomach cancer, Bull. Exp. Biol. Med., 2009, vol. 148, no. 1, p. 82.
Lee, D.G., Jang, S.I., Kim, Y.R., Yang, K.E., Yoon, S.J., Lee, Z.W., An, H.J., Jang, I.S., Choi, J.S., and Yoo, H.S., Anti-proliferative effects of ginsenosides extracted from mountain ginseng on lung cancer, Chin. J. Integr. Med., 2016, vol. 22, no. 5, p. 344. https://doi.org/10.1007/s11655-014-1789-8
Phi, L.T.H., Sari, I.N., Wijaya, Y.T., Kim, K.S., Park, K., Cho, A.E., and Kwon, H.Y., Ginsenoside Rd inhibits the metastasis of colorectal cancer via epidermal growth factor receptor signaling axis, IUBMB Life, 2019, vol. 71, no. 5, p. 601. https://doi.org/10.1002/iub.1984
Zhou, X., Liu, H., Zhang, M., Li, C., and Li, G., Spectrum-effect relationship between UPLC fingerprints and anti-lung cancer effect of Panax ginseng, Phytochem. Anal., 2020, vol. 32, no. 3, p. 339. https://doi.org/10.1002/pca.2980
Liu, G.M., Lu, T.C., Sun, M.L., Jia, W.Y., Ji, X., and Luo, Y.G., Ginsenoside Rd inhibits glioblastoma cell proliferation by up-regulating the expression of miR-144-5p, Biol. Pharm. Bull., 2020, vol. 43, no. 10, p. 1534. https://doi.org/10.1248/bpb.b20-00338
Zhang, E., Shi, H., Yang, L., Wu, X., and Wang, Z., Ginsenoside Rd regulates the Akt/mTOR/p70S6K signaling cascade and suppresses angiogenesis and breast tumor growth, Oncol. Rep., 2017, vol. 38, no. 1, p. 359. https://doi.org/10.3892/or.2017.5652
Zheng, S.W., Xiao, S.Y., Wang, J., Hou, W., and Wang, Y.P., Inhibitory effects of ginsenoside Ro on the growth of B16F10 melanoma via its metabolites, Molecules, 2019, vol. 24, no. 16, p. 2985. https://doi.org/10.3390/molecules24162985
Chian, S., Zhao, Y., Xu, M., Yu, X., Ke, X., Gao, R., and Yin, L., Ginsenoside Rd reverses cisplatin resistance in non-small-cell lung cancer A549 cells by downregulating the nuclear factor erythroid 2-related factor 2 pathway, Anti-Cancer Drugs, 2019, vol. 30, no. 8, p. 838. https://doi.org/10.1097/CAD.0000000000000781
Wang, J., Wang, H., Mou, X., Luan, M., Zhang, X., He, X., Zhao, F., and Meng, Q., The advances on the protective effects of ginsenosides on myocardial ischemia and ischemia-reperfusion injury, Mini-Rev. Med. Chem., 2020, vol. 20, no. 16, p. 1610. https://doi.org/10.2174/1389557520666200619115444
Bai, L., Gao, J., Wei, F., Zhao, J., Wang, D., and Wei, J., Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes, Front. Pharmacol., 2018, vol. 9, p. 423. https://doi.org/10.3389/fphar.2018.00423
Funding
This work was supported in part by grants from the Commission on Biomedical Innovations and Technologies of the Ministry of Science of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Kazeev, I.V., Bocharova, O.A., Shevchenko, V.E. et al. Tandem Mass Spectrometry for the Analysis of Ginsenosides in a Phytoadaptogene Composition with Antitumor Properties. Theor Found Chem Eng 55, 1246–1257 (2021). https://doi.org/10.1134/S0040579521050225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521050225