Abstract
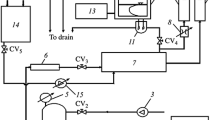
An improved approach to determining the kinetic characteristics of electrochemical pressure-driven membrane separation of solutions has been proposed. It is based on the Spiegler friction theory and takes into account the combination of the effects of chemical and electrochemical potentials. The numerical values of the coefficients of friction fωm, f+m, and f+ω in the solvent–membrane, solute (cations)–membrane and solute (cations)–solvent systems, respectively, were found using the example of the electrochemical pressure-driven membrane separation of aqueous solutions of CuSO4, Ni(NO3)2, and Fe(NO3)3 with concentrations of 1 × 10–2, 2 × 10–3, and 1 × 10–5 mol/L, respectively, using MGA-95 and MGA-100 membranes. Empirical coefficients to determine approximating curves were also determined. It was detected that the absolute values of these coefficients increased with increasing applied electric potential in almost all cases. An exception was mass transfer through the near-cathode membranes in the separation of the Fe(NO3)3 solution. The absolute values of the coefficients of friction were lowest in the separation of the CuSO4 solution and highest in the separation of the Fe(NO3)3 solution. The derived approximating dependences of the coefficients of friction on the electric potential were used to solve the inverse problem to find the retention factors and the outlet solvent flux. This can be efficiently used to predict the mass-transfer mechanism and calculate the characteristics of electromembrane units.






Similar content being viewed by others
REFERENCES
Pushkareva, T.P., Mathematical modeling as a necessary component of mathematical education, Sovrem. Probl. Nauki Obraz., 2014, no. 5, p. 796.
Nikonenko, V.V., Pis’menskaya, N.D., Pourcelly, G., and Larchet, C., Modeling of transport phenomena in systems with ion-exchange membranes, Membrany i membrannye tekhnologii (Membranes and Membrane Technologies), Yaroslavtsev, A.B., Ed., Moscow: Nauchnyi Mir, 2013, ch. 7.
Uzdenova, A.M., Modeling of electroconvection in membrane systems: Analysis of boundary conditions near the surface, Fundam. Issled., 2016, no. 12 (5), p. 1010.
Zabolotskii, V.I., Bugakov, V.V., Sharafan, M.V., and Chermit, R.Kh., Transfer of electrolyte ions and water dissociation in anion-exchange membranes under intense current conditions, Russ. J. Electrochem., 2012, vol. 48, no. 6, pp. 650–659. https://doi.org/10.1134/S1023193512060158
Miękisz, J., Gomułkiewicz, J., and Miękisz, S., Mathematical models of ion transport through cell membrane channels, Math. Appl., 2014, vol. 42, no. 1, p. 39. https://doi.org/10.14708/ma.v42i1.469
Bhadauria, R. and Aluru, N.R., Multiscale modeling of electroosmotic flow: Effects of discrete ion, enhanced viscosity, and surface friction, J. Chem. Phys., 2017, vol. 146, no. 18, p. 184106. https://doi.org/10.1063/1.4982731
Zabolotskii, V.I., Lebedev, K.A., Urtenov, M.K., Nikonenko, V.V., Vasilenko, P.A., Shaposhnik, V.A., and Vasil’eva, V.I., A mathematical model describing voltammograms and transport numbers under intensive electrodialysis modes, Russ. J. Electrochem., 2013, vol. 49, no. 4, pp. 369–380. https://doi.org/10.1134/S1023193513040149
Vorotyntsev, V.M., Drozdov, P.N., and Vorotyntsev, I.V., Mathematical modeling of the fine purification of gas mixtures by absorption pervaporation, Theor. Found. Chem. Eng., 2011, vol. 45, no. 2, pp. 180–184. https://doi.org/10.1134/S0040579511020163
Migol, V.G., Khamizov, R.Kh., Askerniya, A.A., and Korabelnikov, V.M., Effect of precoat filtration and sorption on the mass transfer of silicon-containing compounds through reverse osmosis membranes: I. The model of the process, Sorbtsionnye Khromatogr. Protsessy, 2011, vol. 11, no. 6, pp. 865–872.
Zabolotskii, V.I., Lebedev, K.A., and Shel’deshov, N.V., Ion-transfer across a membrane in the presence of a preceding slow homogeneous chemical reaction in the diffusion layer, Russ. J. Electrochem., 2017, vol. 53, no. 9, pp. 966–979. https://doi.org/10.1134/S102319351709018X
Antipov, S.T. and Klyuchnikov, A.I., Mathematical modeling of microfiltration in a rectangular channel, Theor. Found. Chem. Eng., 2019, vol. 53, no. 1, pp. 83–96. https://doi.org/10.1134/S0040579518060027
Turaev, D.Yu., Use of membrane electrolysis for recovery of heavy metal ions, Russ. J. Appl. Chem., 2007, vol. 80, no. 1, pp. 83–86. https://doi.org/10.1134/S107042720701017X
Vinther, F., Pinelo, M., Brøns, M., Jonsson, G., and Meyer, A.S., Statistical modelling of the interplay between solute shape and rejection in porous membranes, Sep. Purif. Technol., 2012, vol. 89, pp. 261–269. https://doi.org/10.1016/j.seppur.2012.01.032
Kruglikov, S.S., Turaev, D.Yu., and Buzikova, A.M., Regeneration of a copper etching solution used in the manufacture of printed circuit boards by membrane electrolysis, Gal’vanotekh. Obrab. Poverkhn., 2009, vol. 17, no. 1, pp. 59–65.
Unije, U.V., Mücke, R., Baumann, S., and Guillon, O., Comparison of the simplification of the pressure profiles solving the binary friction model for asymmetric membranes, Membranes (Basel, Switz.), 2017, vol. 7, no. 4, p. 58. https://doi.org/10.3390/membranes7040058
Metaiche, M. and Sanchez-Marcano, J., Theoretical considerations of pressure drop and mass transfer of gas flow in spiral wound membrane modules, Int. J. Membr. Sci. Technol., 2016, vol. 3, no. 1, pp. 12–21. https://doi.org/10.15379/2410-1869.2016.03.01.02
Mulder, M., Basic Principles of Membrane Technology, Dordrecht: Kluwer Academic, 1996, 2nd ed.
Zabolotskii, V.I. and Nikonenko, V.V., Perenos ionov v membranakh (Ion Transport in Membranes), Moscow: Nauka, 1996.
Bird, R.B., Stewart, W.E., and Lightfoot, E.N., Transport Phenomena, New York: Wiley, 1960.
Kesting, R.E., Synthetic Polymeric Membranes: A Structural Perspective, New York: Wiley-Interscience, 1985, 2nd ed.
Chalykh, A.E., Diffuziya v polimernykh sistemakh (Diffusion in Polymer Systems), Moscow: Khimiya, 1987.
Nikolaev, N.I., Diffuziya v membranakh (Diffusion in Membranes), Moscow: Khimiya, 1980.
Badessa, T.S., Transport of multiply charged ions through ion-exchange membranes in electrodialysis, Cand. Sci. (Chem.) Dissertation, Voronezh: Voronezh State Univ., 2015.
Verezhnikov, V.N., Praktikum po kolloidnoi khimii poverkhnostno-aktivnykh veshchestv (Practical Work on the Colloid Chemistry of Surface-Active Substances), Voronezh: Voronezh. Gos. Univ., 1984.
Lazarev, S.I., Kovalev, S.V., and Kazakov, V.G., Study of the kinetic and structural characteristics of electrobaromembrane purification of rinsing water from 2,2'-dibenzothiazolyl disulfide production, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2016, vol. 59, no. 2, pp. 34–40.
Kovalev, S.V., Lazarev, S.I., Lazarev, K.S., and Popov, R.V., Flux and rejection coefficient for the MGA-95 membrane in the electrobaromembrane separation of an aqueous solution of zinc sulfate, Vestn. Tambov. Gos. Tekh. Univ., 2015, vol. 21, no. 1, p. 112.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 20–38–90024.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Shestakov, K.V., Lazarev, S.I., Khokhlov, P.A. et al. Predicting the Electrochemical Pressure-Driven Membrane Separation of Industrial Solutions Using Friction Theory. Theor Found Chem Eng 55, 1221–1230 (2021). https://doi.org/10.1134/S0040579521050304
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521050304