Abstract
Background
Upper gastrointestinal (GI) symptoms are common in pediatrics, and few prokinetics for children exist. The goal of this study was to determine the efficacy of prucalopride for treatment of upper GI symptoms and feeding difficulties in children.
Methods
This was a retrospective study of patients prescribed prucalopride for upper GI symptoms at a single tertiary care center from July 2019 to January 2021. Demographic data, the indication for prucalopride, comorbidities, and feeding data were recorded. The primary outcome was improvement in the primary upper GI symptom at first follow-up after prucalopride initiation. Univariable and multivariable analyses were used to assess for factors associated with improvement.
Results
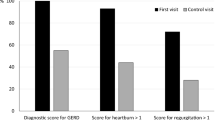
The final study population included 71 patients who received prucalopride for treatment of upper GI symptoms. The most common indications were nausea (44%), feeding difficulties (20%), and reflux (11%). Patients had a median age of 16.7 years (range 1.9–21.8 years), and they had received 4 ± 4.8 years of care in our GI clinic and trialed 3.0 ± 2.0 other GI medications by the time of the prucalopride prescription. At follow-up 3.6 ± 2.9 months after the prucalopride was prescribed, 46 patients (65%) had symptomatic improvement of the upper GI symptom. Improvement was more likely in patients with enteral tubes (p = 0.04), pulmonary comorbidities (p = 0.006), and neurologic comorbidities (p = 0.02). Amongst patients with feeding difficulties, 79% of patients showed improvements in oral or tube feeding.
Conclusions
In this sample of children treated for refractory upper GI symptoms at a single tertiary care center, patients showed improvements in symptoms like nausea, reflux, and feeding difficulties after starting prucalopride.

Similar content being viewed by others
References
MacLennan S, Augood C, Cash-Gibson L, Logan S, Gilbert RE. Cisapride treatment for gastro-oesophageal reflux in children. Cochrane Upper GI and Pancreatic Diseases Group, editor. Cochrane Database Syst Rev. 2010. https://doi.org/10.1002/14651858.CD002300.pub2.
Karunanayake A, Devanarayana NM, de Silva A, Gunawardena S, Rajindrajith S. Randomized controlled clinical trial on value of domperidone in functional abdominal pain in children. J Pediatr Gastroenterol Nutr. 2018;66:725–31.
Tillman EM, Smetana KS, Bantu L, Buckley MG. Pharmacologic treatment for pediatric gastroparesis: a review of the literature. J Pediatr Pharmacol Ther. 2016;21:120–32.
Mani J, Madani S, Thomas R. Economic impact and prognostic factors of functional dyspepsia in children. J Pediatr Gastroenterol Nutr. 2020;70:e65-70.
Lu PL, Moore-Clingenpeel M, Yacob D, Di Lorenzo C, Mousa HM. The rising cost of hospital care for children with gastroparesis: 2004–2013. Neurogastroenterol Motil. 2016;28:1698–704.
Sdravou K. Children with diseases of the upper gastrointestinal tract are more likely to develop feeding problems. Ann Gastroenterol. 2019. Cited 3 Jan 2020. http://www.annalsgastro.gr/files/journals/1/earlyview/2019/ev-01-2019-12-AG4329-0348.pdf
Zangen T, Ciarla C, Zangen S, Di Lorenzo C, Flores AF, Cocjin J, et al. Gastrointestinal motility and sensory abnormalities may contribute to food refusal in medically fragile toddlers. J Pediatr Gastroenterol Nutr. 2003;37:287–93.
Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, et al. Systematic review: cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35:745–67.
Mendzelevski B, Ausma J, Chanter DO, Robinson P, Kerstens R, Vandeplassche L, et al. Assessment of the cardiac safety of prucalopride in healthy volunteers: a randomized, double-blind, placebo- and positive-controlled thorough QT study: Prucalopride thorough QT study in healthy volunteers. Br J Clin Pharmacol. 2012;73:203–9.
Gilsenan A, Fortuny J, Cainzos-Achirica M, Cantero OF, Flynn RWV, Garcia-Rodriguez L, et al. Cardiovascular safety of prucalopride in patients with chronic constipation: a multinational population-based cohort study. Drug Saf. 2019;42:1179–90.
De Maeyer JH, Lefebvre RA, Schuurkes JAJ. 5-HT4 receptor agonists: similar but not the same: 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112.
Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–60.
Kessing BF, Smout AJPM, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil. 2014;26:1079–86.
Carbone F, Van den Houte K, Clevers E, Andrews CN, Papathanasopoulos A, Holvoet L, et al. Prucalopride in gastroparesis: a randomized placebo-controlled crossover study. Am J Gastroenterol. 2019;114:1265–74.
Andrews CN, Woo M, Buresi M, Curley M, Gupta M, Tack J, et al. Prucalopride in diabetic and connective tissue disease-related gastroparesis: Randomized placebo-controlled crossover pilot trial. Neurogastroenterol Motil. 2021. https://doi.org/10.1111/nmo.13958.
Nennstiel S, Bajbouj M, Schmid RM, Becker V. Prucalopride reduces the number of reflux episodes and improves subjective symptoms in gastroesophageal reflux disease: a case series. J Med Case Reports. 2014;8:34.
Yi C-H, Lei W-Y, Hung J-S, Liu T-T, Chen C-L. Effects of prucalopride on esophageal secondary peristalsis in humans. Clin Transl Gastroenterol. 2016;7:202.
Lei W-Y, Hung J-S, Liu T-T, Yi C-H, Chen C-L. Influence of prucalopride on esophageal secondary peristalsis in reflux patients with ineffective motility: prucalopride and secondary peristalsis. J Gastroenterol Hepatol. 2018;33:650–5.
Ng TSC, Putta N, Kwatra NS, Drubach LA, Rosen R, Fahey FH, et al. Pediatric solid gastric emptying scintigraphy: normative value guidelines and nonstandard meal alternatives. Am J Gastroenterol. 2020;115:1830–9.
Kwatra NS, Shalaby-Rana E, Andrich MP, Tsai J, Rice AL, Ghelani SJ, et al. Gastric emptying of milk in infants and children up to 5 years of age: normative data and influencing factors. Pediatr Radiol. 2020;50:689–97.
McVeagh P. Pulmonary aspiration studied by radionuclide milk scanning and barium swallow roentgenography. Arch Pediatr Adolesc Med. 1987;141:917.
Rosen R, Garza JM, Tipnis N, Nurko S. An ANMS-NASPGHAN consensus document on esophageal and antroduodenal manometry in children. Neurogastroenterol Motil. 2018;30:13239.
Mougey EB, Saunders M, Franciosi JP, Gomez-Suarez RA. Comparative effectiveness of IV azithromycin vs erythromycin stimulating antroduodenal motility in children. J Pediatr Gastroenterol Nutr. 2021. https://doi.org/10.1097/MPG.0000000000003271 ([cited 2021 Oct 29];Publish Ahead of Print).
Di Lorenzo C, Lucanto C, Flores A, Idries S, Hyman PE. Effect of sequential erythromycin and octreotide on antroduodenal manometry. J Pediatr Gastroenterol Nutr. 1999;29:293–6.
Damrongmanee A, El-Chammas K, Fei L, Liu C, Santucci N, Kaul A. Effects of provocative testing on phase iii migrating motor complex in children. J Pediatr Gastroenterol Nutr. 2021;73:507–12.
Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38.
Emmanuel AV, Kamm MA, Roy AJ, Kerstens R, Vandeplassche L. Randomised clinical trial: the efficacy of prucalopride in patients with chronic intestinal pseudo-obstruction—a double-blind, placebo-controlled, cross-over, multiple n = 1 study: Randomised clinical trial: prucalopride in chronic intestinal pseudo-obstruction. Aliment Pharmacol Ther. 2012;35:48–55.
Parkman HP, Camilleri M, Farrugia G, McCallum RW, Bharucha AE, Mayer EA, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113–33.
Lu PL, Di Lorenzo C. Gastroparesis in the pediatric patient: children are not little adults. Gastrointest Disord. 2020;2:86–95.
Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783–8.
Kawahara H, Tazuke Y, Soh H, Usui N, Okuyama H. Characteristics of gastroesophageal reflux in pediatric patients with neurological impairment. Pediatr Surg Int. 2017;33:1073–9.
Sullivan PB, Lambert B, Rose M, Ford-Adams M, Johnson A, Griffiths P. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev Med Child Neurol. 2000;42:674–80.
Ambartsumyan L, Nurko S, Rosen R. Gastrointestinal dysmotility and the implications for respiratory disease. Curr Treat Options Pediatr. 2019;5:197–214.
Winter HS, Di Lorenzo C, Benninga MA, Gilger MA, Kearns GL, Hyman PE, et al. Oral prucalopride in children with functional constipation. J Pediatr Gastroenterol Nutr. 2013;57:197–203.
Mugie SM, Korczowski B, Bodi P, Green A, Kerstens R, Ausma J, et al. Prucalopride is no more effective than placebo for children with functional constipation. Gastroenterology. 2014;147:1285-1295.e1.
Nurko S, Saps M. Treating constipation with prucalopride: one size does not fit all. Gastroenterology. 2014;147:1214–6.
Leelakusolvong S, Ke M, Zou D, Choi SC, Tack J, Quigley EMM, et al. Factors predictive of treatment-emergent adverse events of prucalopride: an integrated analysis of four randomized, double-blind. Placebo-Controlled Trials Gut Liver. 2015;9:208–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Supported by National Institutes of Health R01 DK097112-01 and T32 DK007477-37.
Conflict of interest
Suzanna Hirsch, Samuel Nurko, Paul Mitchell, and Rachel Rosen declare that they have no conflicts of interest.
Ethics approval
Institutional review board approval was obtained for this retrospective chart review. The study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Consent to participate
Consent to participate was not required in this study as it was a retrospective chart review. All data analysis was performed in a de-identified fashion.
Consent for publication
Consent for publication was not required in this study as it was a retrospective chart review.
Availability of data and material
The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request.
Code availability
The code utilized for analysis of the current study is available from the corresponding author on reasonable request.
Author contributions
SH contributed to the conceptualization of this study, the methodology and investigation, formal analysis, and drafting and editing of the manuscript. SN contributed to the conceptualization and investigation of the study and to manuscript review and editing. PM contributed to formal analysis and interpretation of data and to manuscript review and editing. RR contributed to the conceptualization of this study, the methodology and investigation, supervision and oversight of the study, and to manuscript review and editing. All authors revised and approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rights and permissions
About this article
Cite this article
Hirsch, S., Nurko, S., Mitchell, P. et al. Prucalopride for Treatment of Upper Gastrointestinal Symptoms in Children. Pediatr Drugs 24, 73–81 (2022). https://doi.org/10.1007/s40272-021-00489-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00489-5