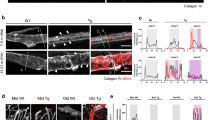
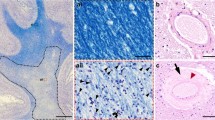
Abstract
Perivascular spaces (PVS) are compartments surrounding cerebral blood vessels that become visible on MRI when enlarged. Enlarged PVS (EPVS) are commonly seen in patients with cerebral small vessel disease (CSVD) and have been suggested to reflect dysfunctional perivascular clearance of soluble waste products from the brain. In this study, we investigated histopathological correlates of EPVS and how they relate to vascular amyloid-β (Aβ) in cerebral amyloid angiopathy (CAA), a form of CSVD that commonly co-exists with Alzheimer’s disease (AD) pathology. We used ex vivo MRI, semi-automatic segmentation and validated deep-learning-based models to quantify EPVS and associated histopathological abnormalities. Severity of MRI-visible PVS during life was significantly associated with severity of MRI-visible PVS on ex vivo MRI in formalin fixed intact hemispheres and corresponded with PVS enlargement on histopathology in the same areas. EPVS were located mainly around the white matter portion of perforating cortical arterioles and their burden was associated with CAA severity in the overlying cortex. Furthermore, we observed markedly reduced smooth muscle cells and increased vascular Aβ accumulation, extending into the WM, in individually affected vessels with an EPVS. Overall, these findings are consistent with the notion that EPVS reflect impaired outward flow along arterioles and have implications for our understanding of perivascular clearance mechanisms, which play an important role in the pathophysiology of CAA and AD.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG (2018) The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135:387–407. https://doi.org/10.1007/s00401-018-1812-4
Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G (2019) Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci 11:1–17. https://doi.org/10.3389/fnagi.2019.00001
Atzeni A, Jansen M, Ourselin S, Iglesias JE (2018) A probabilistic model combining deep learning and multi-atlas segmentation for semi-automated labelling of histology. Lect Notes Comput Sci 11071:219–227. https://doi.org/10.1007/978-3-030-00934-2_25
Bacyinski A, Xu M, Wang W, Hu J (2017) The paravascular pathway for brain waste clearance: current understanding, significance and controversy. Front Neuroanat 11:1–8. https://doi.org/10.3389/fnana.2017.00101
Banerjee G, Kim HJ, Fox Z, Jäger HR, Wilson D, Charidimou A et al (2017) MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain 140:1107–1116. https://doi.org/10.1093/brain/awx003
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J (2019) The glymphatic system and waste clearance with brain aging: a review. Gerontology 65:106–119. https://doi.org/10.1159/000490349
Benveniste H, Nedergaard M (2022) Cerebral small vessel disease: a glymphopathy? Curr Opin Neurobiol 72:15–21. https://doi.org/10.1016/j.conb.2021.07.006
Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM et al (2008) Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain 131:3299–3310. https://doi.org/10.1093/brain/awn261
Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJM (2014) Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Invest Radiol 49:307–313. https://doi.org/10.1097/RLI.0000000000000027
Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA (2018) Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 83:74–83. https://doi.org/10.1002/ana.25123
Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A et al (2018) Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 114:1462–1473. https://doi.org/10.1093/cvr/cvy113
Carare RO, Aldea R, Bulters D, Alzetani A, Birch AA, Richardson G et al (2020) Vasomotion drives periarterial drainage of Aβ from the brain. Neuron 105:400–401. https://doi.org/10.1016/j.neuron.2020.01.011
Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JAR, Perry VH et al (2008) Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34:131–144. https://doi.org/10.1111/j.1365-2990.2007.00926.x
Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP et al (2017) Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. https://doi.org/10.1093/brain/awx047
Charidimou A, Boulouis G, Pasi M, Auriel E, Van Etten ES, Haley K et al (2017) MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 88:1157–1164. https://doi.org/10.1212/WNL.0000000000003746
Charidimou A, Hong YT, Jäger HR, Fox Z, Aigbirhio FI, Fryer TD et al (2015) White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke 46:1707–1709. https://doi.org/10.1161/STROKEAHA.115.009090
Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A et al (2014) White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology 82:57–62. https://doi.org/10.1212/01.wnl.0000438225.02729.04
Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P et al (2013) Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry 84:624–629. https://doi.org/10.1136/jnnp-2012-304434
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3D U-net: learning dense volumetric segmentation from sparse annotation. arXiv:160606650v1 [csCV]. https://doi.org/10.1007/978-3-319-46723-8_49
Clevert DA, Unterthiner T, Hochreiter S (2016) Fast and accurate deep network learning by Exponential linear units (ELUs). arXiv:151107289v5 [csLG]
Diem AK, Sharp MMG, Gatherer M, Bressloff NW, Carare RO, Richardson G (2017) Arterial pulsations cannot drive intramural periarterial drainage: significance for Aβ drainage. Front Neurosci 11:1–9. https://doi.org/10.3389/fnins.2017.00475
Dubost F, Yilmaz P, Adams H, Bortsova G, Ikram MA, Niessen W et al (2019) Enlarged perivascular spaces in brain MRI: automated quantification in four regions. Neuroimage 185:534–544. https://doi.org/10.1016/j.neuroimage.2018.10.026
Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D et al (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, part xv. Am Acad Neurol 46:1592–1596. https://doi.org/10.1111/j.1532-5415.1997.tb00968.x
Francis F, Ballerini L, Wardlaw JM (2018) Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: a systematic review and meta-analysis. Int J Stroke 14:359–371. https://doi.org/10.1177/1747493019830321
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ (2020) Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol 16:30–42. https://doi.org/10.1038/s41582-019-0281-2
Grinberg LT, Thal DR (2010) Vascular pathology in the aged human brain. Acta Neuropathol 119:277–290. https://doi.org/10.1007/s00401-010-0652-7
Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA et al (2011) Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121:431–443. https://doi.org/10.1007/s00401-011-0801-7
Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO (2014) Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy. Brain Pathol 24:396–403. https://doi.org/10.1111/bpa.12159
Hou Y, Park SH, Wang Q, Zhang J, Zong X, Lin W et al (2017) Enhancement of perivascular spaces in 7 T MR image using haar transform of non-local cubes and block-matching filtering. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-09336-5
Iglesias JE, Sabuncu MR (2015) Multi-atlas segmentation of biomedical images: a survey. Med Image Anal 24:205–219. https://doi.org/10.1016/j.media.2015.06.012
Iliff JJ, Nedergaard M, Lee H, Yu M, Benveniste H, Feng T et al (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123(3):1299–1309. https://doi.org/10.1172/JCI67677
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Weiner MW et al (2016) Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7:11934. https://doi.org/10.1038/ncomms11934
Jäkel L, De Kort AM, Klijn CJM, Schreuder FHBM, Verbeek MM (2021) Prevalence of cerebral amyloid angiopathy: a systematic review and meta-analysis. Alzheimer’s Dement. https://doi.org/10.1002/alz.12366
Javierre-Petit C, Schneider JA, Kapasi A, Makkinejad N, Tamhane AA, Leurgans SE et al (2020) Neuropathologic and cognitive correlates of enlarged perivascular spaces in a community-based cohort of older adults. Stroke 51:2825–2833. https://doi.org/10.1161/STROKEAHA.120.029388
Jochems ACC, Blair GW, Stringer MS, Thrippleton MJ, Clancy U, Chappell FM et al (2020) Relationship between venules and perivascular spaces in sporadic small vessel diseases. Stroke 51:1503–1506. https://doi.org/10.1161/STROKEAHA.120.029163
Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J et al (2016) Ultra-fast magnetic resonance encephalography of physiological brain activity-Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36(6):1033–1045. https://doi.org/10.1177/0271678X15622047
Kövari E, Herrmann FR, Gold G, Hof PR, Charidimou A (2017) Association of cortical microinfarcts and cerebral small vessel pathology in the ageing brain. Neuropathol Appl Neurobiol 43:505–513. https://doi.org/10.1111/nan.12366
Kress BT, Iliff JJ, Xia M, Wang M, Wei H et al (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. https://doi.org/10.1002/ana.24271.Impairment
Lei Y, Han H, Yuan F, Javeed A, Zhao Y (2017) The brain interstitial system: anatomy, modeling, in vivo measurement, and applications. Prog Neurobiol 157:230–246. https://doi.org/10.1016/j.pneurobio.2015.12.007
Maat-schieman M, Roos R, Van DS (2005) Hereditary cerebral hemorrhage with amyloidosis- Dutch type. Neuropathology 25:288–297. https://doi.org/10.1016/j.nbd.2003.08.019
MacGregor Sharp M, Bulters D, Brandner S, Holton J, Verma A, Werring DJ et al (2019) The fine anatomy of the perivascular compartment in the human brain: relevance to dilated perivascular spaces in cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 45(3):305–308. https://doi.org/10.1111/nan.12480
Martinez-Ramirez S, Van Rooden S, Charidimou A, Van Opstal AM, Wermer M, Edip Gurol M et al (2018) Perivascular spaces volume in sporadic and hereditary (Dutch-type) cerebral amyloid angiopathy. Stroke 49:1913–1919. https://doi.org/10.1161/STROKEAHA.118.021137
Mestre H, Kostrikov S, Mehta RI, Nedergaard M (2017) Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci 131(17):2257–2274. https://doi.org/10.1042/cs20160381
Milletari F, Navab N, Ahmadi SA (2016) V-Net: fully convolutional neural networks for volumetric medical image segmentation. arXiv:160604797v1 [csCV]. https://doi.org/10.1109/3DV.2016.79
Minckler J (1968) Vascular tissues in the central nervous system. Pathology of the nervous system. McGraw-Hill, New York, pp 486–498
Miyata M, Kakeda S, Iwata S, Nakayamada S, Ide S, Watanabe K et al (2017) Enlarged perivascular spaces are associated with the disease activity in systemic lupus erythematosus. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-12966-4
Perosa V, Scherlek AA, Kozberg MG, Smith L, Bui TW, Auger CA et al (2021) Deep learning assisted quantitative assessment of histopathological markers of Alzheimer’s disease and cerebral amyloid angiopathy. Acta Neuropathol Commun 9(1):141. https://doi.org/10.1186/s40478-021-01235-1
Pollock H, Hutchings M, Weller RO, Zhang ET (1997) Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat 191:337–346. https://doi.org/10.1017/S0021878297002458
Potter GM, Chappell FM, Morris Z, Wardlaw JM (2015) Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis 39:224–231. https://doi.org/10.1159/000375153
Power MC, Mormino E, Soldan A, James BD, Yu L, Armstrong NM et al (2018) Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol 84:10–22. https://doi.org/10.1002/ana.25246
Raposo N, Planton M, Payoux P, Péran P, Albucher JF, Calviere L et al (2019) Enlarged perivascular spaces and florbetapir uptake in patients with intracerebral hemorrhage. Eur J Nucl Med Mol Imaging 46:2339–2347. https://doi.org/10.1007/s00259-019-04441-1
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024. https://doi.org/10.1016/S1474-4422(18)30318-1
Reuter M, Rosas HD, Fischl B (2010) Highly accurate inverse consistent registration: a robust approach. Neuroimage 53:1181–1196. https://doi.org/10.1016/j.neuroimage.2010.07.020
Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W et al (2003) Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med 9:112–122. https://doi.org/10.1007/bf03402043
Rohlfing T, Brandt R, Menzel R, Maurer CR (2004) Evaluation of atlas selection strategies for atlas-based image segmentation with application to confocal microscopy images of bee brains. Neuroimage 21:1428–1442. https://doi.org/10.1016/j.neuroimage.2003.11.010
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. arXiv:150504597 [csCV]. https://doi.org/10.1109/ACCESS.2021.3053408
Satizabal CL, Zhu YC, Dufouil C, Tzourio C (2013) Inflammatory proteins and the severity of dilated Virchow-Robin spaces in the elderly. J Alzheimer’s Dis 33:323–328. https://doi.org/10.3233/JAD-2012-120874
Schindelin J, Arganda-Carrera I, Frise E, Verena K, Mark L, Tobias P et al (2012) Fiji—an Open platform for biological image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019.Fiji
Sepehrband F, Barisano G, Sheikh-Bahaei N, Cabeen RP, Choupan J, Law M et al (2019) Image processing approaches to enhance perivascular space visibility and quantification using MRI. Sci Rep 9:12351. https://doi.org/10.1038/s41598-019-48910-x
Shams S, Martola J, Charidimou A, Larvie M, Granberg T, Shams M et al (2017) Topography and determinants of Magnetic resonance imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 6:1–8. https://doi.org/10.1161/JAHA.117.006279
Swieten JCV, Den HJHWV, Ketel BAV, Hijdra A, Wokke JHJ, Van GJ (1991) Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 114:761–774. https://doi.org/10.1093/brain/114.2.761
Takahashi S (2010) Neurovascular Imaging. Springer-Verlag, London limited
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E et al (2015) Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol 11(8):457–470. https://doi.org/10.1038/nrneurol.2015.119
Van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WGM, Zwanenburg JJM, Luijten PR et al (2016) Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab 36(3):576–580. https://doi.org/10.1177/0271678X15620434
van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP et al (2019) Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105:549–561. https://doi.org/10.1016/j.neuron.2019.10.033
van Veluw SJ, Scherlek AA, Freeze WM, ter Telgte A, van der Kouwe AJ, Bacskai BJ et al (2019) Different microvascular alterations underlie microbleeds and microinfarcts. Ann Neurol 86(2):279–292. https://doi.org/10.1002/ana.25512
Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q et al (2017) White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging 50:96–106. https://doi.org/10.1016/j.neurobiolaging.2016.11.002
Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ et al (2017) Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci 37:2870–2877. https://doi.org/10.1523/jneurosci.2112-16.2017
Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H et al (2020) Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 16(3):137–153. https://doi.org/10.1038/s41582-020-0312-z
Wardlaw JM, Smith C, Dichgans M (2019) Small vessel disease: mechanisms and clinical implications. Lancet Neurol 18:684–696. https://doi.org/10.1016/S1474-4422(19)30079-1
Webb AJS, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM (2012) Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke 43:2631–2636. https://doi.org/10.1161/STROKEAHA.112.655837
Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD et al (2008) Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain 131:2332–2340. https://doi.org/10.1093/brain/awn171
Yao M, Hervé D, Jouvent E, Duering M, Reyes S, Godin O et al (2014) Dilated perivascular spaces in small-vessel disease: a study in CADASIL. Cerebrovasc Dis 37:155–163. https://doi.org/10.1159/000356982
Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H (2010) Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 41:2483–2490. https://doi.org/10.1161/STROKEAHA.110.591586
Acknowledgements
We wish to thank Sjoerd van Duinen, Gisela Terwindt, Marieke Wermer, and Louise van der Weerd from Leiden University Medical Center (LUMC) for providing the brain sample of the D-CAA case. Furthermore, we thank the patients and their families for participating in the brain donation program. This work was supported by the Harvard Catalyst, Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding
This work was funded by the National Institutes of Health (AG059893 to S.J.v.V., RF1 NS110054 to B.J.B.; 1RF1MH123195-01 and 1R01AG070988-01 to J.E.I.), the German Research Foundation (DFG) (454245528 to V.P.), Alzheimer’s Research UK (ARUK-IRG2019A-003), and the European Research Council (Starting Grant 677697, project “BUNGEE-TOOLS”).
Author information
Authors and Affiliations
Contributions
VP and SJvV designed the study. SMG and AV supported data collection. CAA and AAS processed histopathological material. AJvdK provided support in optimizing the scanning parameters. Data were analyzed by VP with support from JO, JEIG, WMF and AA. VP and SJvV drafted the manuscript and all the authors reviewed it and provided feedback. The project was supervised by SJvV.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MOV 9853 KB)
Supplementary file3 (MOV 5434 KB)
Supplementary file4 (MOV 5043 KB)
Rights and permissions
About this article
Cite this article
Perosa, V., Oltmer, J., Munting, L.P. et al. Perivascular space dilation is associated with vascular amyloid-β accumulation in the overlying cortex. Acta Neuropathol 143, 331–348 (2022). https://doi.org/10.1007/s00401-021-02393-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02393-1