mRNA technology may be uniquely positioned to tackle a major hurdle for HIV vaccines: the elicitation of broadly cross-reactive neutralizing antibodies. A preclinical study takes the first step toward this goal.
The remarkable success of mRNA vaccines against COVID-19 has been nothing short of miraculous. Whether this unique technology platform can be used to tackle the more complex task of developing a vaccine against human immunodeficiency virus (HIV) is now under intense scrutiny. In this issue of Nature Medicine, a preclinical study by Zhang et al. suggests that the mRNA platform may be up to the challenge1. The authors encapsulated mRNA encoding the HIV envelope glycoprotein (Env) (the equivalent of the SARS-CoV-2 spike protein), together with the structural HIV group-specific antigen protein (Gag), in a lipid nanoparticle, to produce virus-like particles (VLPs) in vivo. These Env-expressing VLPs elicited broadly neutralizing antibodies (bNAbs) and other immune responses that were protective against viral challenge in a macaque model. Although the VLPs were not nearly as immunogenic or as efficacious as mRNA vaccines against COVID-19, these results are encouraging and illuminate a pathway toward inducing the higher and more-durable antibody responses needed to prevent infection with HIV.
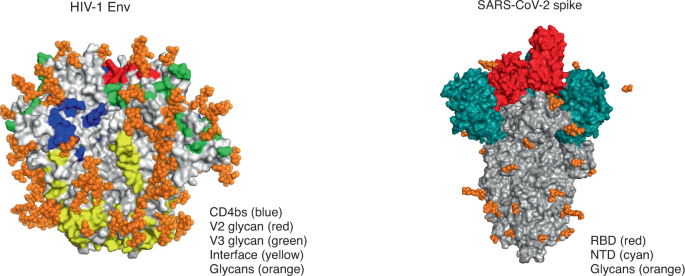
This is no small feat; Env is a formidable target that fails to induce the right kind of antibodies even in the majority of people who are infected with HIV. Unlike spike protein, which is relatively stable and contains just a few immunodominant epitopes (mostly restricted to the receptor-binding domain), Env is a complex trimeric protein with multiple dispersed antibody epitopes — many of which are conformational and heavily coated in sugars that shield them from antibody attack (Fig. 1). The ability to generate soluble trimeric Env proteins through the introduction of key mutations was a major advance in immunogen design, although these so-called ‘SOSIP proteins’ induced only autologous strain–specific neutralizing antibodies2. In the present study by Zhang et al., antibodies elicited by the Env–Gag mRNA were able to neutralize almost all members of a global panel of HIV isolates, classified as having a tier 2 phenotype1. This represents a major step forward for a vaccine against HIV, as this phenotype is typical of most circulating strains, which are difficult to neutralize due to a closed Env conformation.
HIV Env (left) has multiple conformational bNAb epitopes (four of the six epitopes are shown here), is densely covered in glycans, and elicits a polyclonal neutralizing antibody response. Genes encoding antibodies to Env are heavily mutated, and bNAbs are rarely elicited by infection or vaccination. In contrast, the SARS-CoV-2 spike protein (right) is lightly covered in glycans, and the receptor-binding domain (RBD) is the main immunodominant epitope, with the N-terminal domain (NTD) targeted to a lesser extent. It generates a focused neutralizing antibody response; genes encoding antibodies to spike protein have limited or no mutations, and bNAbs are commonly elicited after infection and vaccination. CD4bs, CD4-binding site. Images from RCSB Protein Data Bank (https://www.rcsb.org/) accession codes 4ZMJ (glycans transposed from 5FUU; HIV Env) and 6VSB (glycans transposed from 7CN9; SARS-CoV-2 spike protein). We thank T. Moyo-Gwete for preparation of this figure.
It is likely that a number of factors contributed to the greater immunogenicity of the mRNA vaccine designed by Zhang et al.1. The endogenous expression of the native Env on the surface of a VLP would preserve conformational epitopes, and VLPs were also shown to contain double the number of Env molecules present in an HIV viral particle. Furthermore, mRNA continues to be expressed for several days after administration, providing ongoing immunostimulation3. Another potential advantage is the ability of VLPs to bind to antigen-presenting cells, which guarantees delivery of mRNA into the appropriate cells and the induction of follicular helper T cells that are crucial for B cell development in germinal centers4. Zhang et al. also chose an HIV Env sequence that lacks a glycan at position 276; this enabled better access to the B cell precursors of bNAbs that target the CD4-binding site on the viral envelope1. Indeed, Env–Gag mRNA elicited antibodies to that site and, interestingly, to many other HIV epitopes — a considerable improvement on the strain-specific ‘glycan-hole’ responses seen with SOSIP proteins. It will be important in future studies to isolate B cells from mRNA-immunized animals to ascertain whether they are capable of broad neutralization and if they carry the genetic features associated with known bNAb lineages. Although the results from Zhang et al.1 are encouraging for the HIV vaccine field, this is a complex and impractical protocol that requires multiple immunizations with high doses of mRNA. Moreover, the levels of bNAbs elicited in this study were extremely low and took a year to develop, and their role in protection from infection remains unclear.
mRNA technology has only recently come of age; it was originally hampered by instability and unfavorable immunogenicity, but decades of research have solved these problems, and the advantages of mRNA as a vaccine platform continue to emerge5. These include rapid development, ease of manufacture and scalability, which offer advantages over the traditional vector-based or protein-based vaccines being pursued in the HIV field. For example, mRNA would enable testing of sequential immunization and lineage-based approaches that require multiple immunogens with minor but important sequence changes6. mRNA is also considerably cheaper to produce and can be modified as needed, an important consideration for rapidly mutating viruses like HIV. Among the vaccines against COVID-19, those based on the mRNA platform have superior immunogenicity and stimulate both B cell responses and T cell responses7,8. The precise mechanisms underlying mRNA immunogenicity, however, are still unknown, and much remains to be learned if they are to be optimized and applied to HIV prevention. Nonetheless, the vast amount of safety data available from mRNA vaccines against COVID-19 will probably contribute to streamlined regulatory approval processes for vaccines against HIV and other diseases.
In contrast to the swift success of vaccines against COVID-19, the story of the development of vaccines against HIV has been long and troubled. Of the eight vaccine efficacy trials conducted so far, only one (with a viral-vector and protein-based vaccine prime–boost regimen) has shown moderate efficacy, although the follow-up trial failed to replicate this result9. This has shifted the field back to the pursuit of a vaccine able to induce bNAbs. Recently, the Antibody-Mediated Prevention clinical trials demonstrated that a monoclonal bNAb against the CD4-binding site can prevent infection of humans with HIV10, although it came with the sobering realization that high levels of bNAbs will probably be required (P. Gilbert, personal communication). Another approach to generating bNAbs is to trigger the B cell precursors of specific bNAb lineages. One such germline-targeting immunogen is eOD-GT8, a nanoparticle coated with HIV Env gp120 proteins, which binds rare B cells specific for the CD4-binding site in monkeys and humans11. eOD-GT8 has now been converted into an mRNA vaccine through the same platform as the successful Moderna vaccine against COVID-19, with human clinical trials due to start soon.
There is no doubt that HIV presents a much greater challenge for vaccine developers than COVID-19 does. The vast genetic diversity and the ability of HIV to integrate into the human genome necessities that a vaccine elicits antibodies able to block every viral particle. Whether further optimization of Env immunogens, together with the strong priming effect of an mRNA vaccine platform, is able to achieve this will require further investigation. The hope is that the lessons learned from the development of vaccines against COVID-19 will be used to solve the HIV problem and that this will be tackled with the same sense of urgency, given that HIV remains a major global health challenge.
References
Zhang. P. et al. Nat. Med. https://doi.org/10.1038/s41591-021-01574-5 (2021).
Sanders, R. W. et al. Science 349, aac4223 (2015).
Pardi, N. et al. J. Control. Release 217, 345–351 (2015).
Pardi, N. et al. J. Exp. Med. 215, 1571–1588 (2018).
Chaudhary, N., Weissman, D. & Whitehead, K. A. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Mu, Z., Haynes, B. F. & Cain, D. W. Vaccines 9, 134 (2021).
Khoury, D. S. et al. Nat. Med. 27, 1205–1211 (2021).
Lederer, K. et al. Immunity 53, 1281–1295.e5 (2020).
Gray, G. E. et al. N. Engl. J. Med. 384, 1089–1100 (2021).
Corey, L. et al. N. Engl. J. Med. 384, 1003–1014 (2021).
Jardine, J. G. et al. Science 351, 1458–1463 (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Rights and permissions
About this article
Cite this article
Morris, L. mRNA vaccines offer hope for HIV. Nat Med 27, 2082–2084 (2021). https://doi.org/10.1038/s41591-021-01602-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-021-01602-4