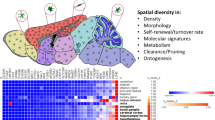
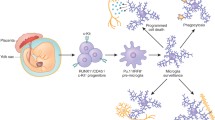
Abstract
Microglia, the resident myeloid cells in the central nervous system (CNS) play critical roles in shaping the brain during development, responding to invading pathogens, and clearing tissue debris or aberrant protein aggregations during ageing and neurodegeneration. The original concept that like macrophages, microglia are either damaging (pro-inflammatory) or regenerative (anti-inflammatory) has been updated to a kaleidoscope view of microglia phenotypes reflecting their wide-ranging roles in maintaining homeostasis in the CNS and, their contribution to CNS diseases, as well as aiding repair. The use of new technologies including single cell/nucleus RNA sequencing has led to the identification of many novel microglia states, allowing for a better understanding of their complexity and distinguishing regional variations in the CNS. This has also revealed differences between species and diseases, and between microglia and other myeloid cells in the CNS. However, most of the data on microglia heterogeneity have been generated on cells isolated from the cortex or whole brain, whereas white matter changes and differences between white and grey matter have been relatively understudied. Considering the importance of microglia in regulating white matter health, we provide a brief update on the current knowledge of microglia heterogeneity in the white matter, how microglia are important for the development of the CNS, and how microglial ageing affects CNS white matter homeostasis. We discuss how microglia are intricately linked to the classical white matter diseases such as multiple sclerosis and genetic white matter diseases, and their putative roles in neurodegenerative diseases in which white matter is also affected. Understanding the wide variety of microglial functions in the white matter may provide the basis for microglial targeted therapies for CNS diseases.




Similar content being viewed by others
References
Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J et al (2021) A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597:709–714. https://doi.org/10.1038/s41586-021-03892-7
Adams SJ, Kirk A, Auer RN (2018) Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP): Integrating the literature on hereditary diffuse leukoencephalopathy with spheroids (HDLS) and pigmentary orthochromatic leukodystrophy (POLD). J Clin Neurosci 48:42–49. https://doi.org/10.1016/j.jocn.2017.10.060
Agarwal M, Biswas P, Bhattacharya A, Sinha DK (2020) Reactive oxygen species-mediated cytoplasmic stiffening impairs the phagocytic ability of the macrophage. J Cell Sci. https://doi.org/10.1242/jcs.236471
Ajami B, Samusik N, Wieghofer P, Ho PP, Crotti A, Bjornson Z et al (2018) Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat Neurosci 21:541–551. https://doi.org/10.1038/s41593-018-0100-x
Allen SJ, Baker D, O’Neill JK, Davison AN, Turk JL (1993) Isolation and characterization of cells infiltrating the spinal cord during the course of chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Cell Immunol 146:335–350. https://doi.org/10.1006/cimm.1993.1031
Amici SA, Dong J, Guerau-de-Arellano M (2017) Molecular mechanisms modulating the phenotype of macrophages and microglia. Front Immunol 8:1520. https://doi.org/10.3389/fimmu.2017.01520
Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P et al (2017) Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep 18:391–405. https://doi.org/10.1016/j.celrep.2016.12.041
Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A et al (2020) Negative feedback control of neuronal activity by microglia. Nature 586:417–423. https://doi.org/10.1038/s41586-020-2777-8
Baker D, Amor S (2015) Mouse models of multiple sclerosis: lost in translation? Curr Pharm Des 21:2440–2452. https://doi.org/10.2174/1381612821666150316122706
Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K (2017) Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurology-Neuroimmunology Neuroinflammation 4
Baror R, Neumann B, Segel M, Chalut KJ, Fancy SPJ, Schafer DP et al (2019) Transforming growth factor-beta renders ageing microglia inhibitory to oligodendrocyte generation by CNS progenitors. Glia 67:1374–1384. https://doi.org/10.1002/glia.23612
Bauer J, Ruuls SR, Huitinga I, Dijkstra CD (1996) The role of macrophage subpopulations in autoimmune disease of the central nervous system. Histochem J 28:83–97. https://doi.org/10.1007/BF02331413
Beckmann N, Giorgetti E, Neuhaus A, Zurbruegg S, Accart N, Smith P et al (2018) Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol Commun 6:9. https://doi.org/10.1186/s40478-018-0510-8
Bellver-Landete V, Bretheau F, Mailhot B, Vallieres N, Lessard M, Janelle ME et al (2019) Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun 10:518. https://doi.org/10.1038/s41467-019-08446-0
Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB et al (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113:E1738-1746. https://doi.org/10.1073/pnas.1525528113
Bergner CG, van der Meer F, Winkler A, Wrzos C, Turkmen M, Valizada E et al (2019) Microglia damage precedes major myelin breakdown in X-linked adrenoleukodystrophy and metachromatic leukodystrophy. Glia 67:1196–1209. https://doi.org/10.1002/glia.23598
Bottcher C, Schlickeiser S, Sneeboer MAM, Kunkel D, Knop A, Paza E et al (2019) Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci 22:78–90. https://doi.org/10.1038/s41593-018-0290-2
Brettschneider J, Toledo JB, Van Deerlin VM, Elman L, McCluskey L, Lee VM et al (2012) Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE 7:e39216. https://doi.org/10.1371/journal.pone.0039216
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G et al (2014) Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17:131–143. https://doi.org/10.1038/nn.3599
Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY et al (2016) Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 18:557–564. https://doi.org/10.1093/neuonc/nov245
Chen JF, Liu K, Hu B, Li RR, Xin W, Chen H et al (2021) Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 109(2292–2307):e2295. https://doi.org/10.1016/j.neuron.2021.05.012
Chen WT, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N et al (2020) Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 182(976–991):e919. https://doi.org/10.1016/j.cell.2020.06.038
Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4:399–418. https://doi.org/10.1007/s11481-009-9164-4
Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E et al (2018) Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med 215:1627–1647. https://doi.org/10.1084/jem.20180247
D’Erchia AM, Gallo A, Manzari C, Raho S, Horner DS, Chiara M et al (2017) Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci Rep 7:10046. https://doi.org/10.1038/s41598-017-10488-7
Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S et al (2002) Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99:111–120. https://doi.org/10.1182/blood.v99.1.111
Dardiotis E, Siokas V, Pantazi E, Dardioti M, Rikos D, Xiromerisiou G et al (2017) A novel mutation in TREM2 gene causing Nasu-Hakola disease and review of the literature. Neurobiol Aging. https://doi.org/10.1016/j.neurobiolaging.2017.01.015
Dawson TM, Golde TE, Lagier-Tourenne C (2018) Animal models of neurodegenerative diseases. Nat Neurosci 21:1370–1379. https://doi.org/10.1038/s41593-018-0236-8
De Biase LM, Bonci A (2019) Region-specific phenotypes of microglia: the role of local regulatory cues. Neuroscientist 25:314–333. https://doi.org/10.1177/1073858418800996
Dols-Icardo O, Montal V, Sirisi S, Lopez-Pernas G, Cervera-Carles L, Querol-Vilaseca M et al (2020) Motor cortex transcriptome reveals microglial key events in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 7:e829. https://doi.org/10.1212/NXI.0000000000000829
Dubbelaar ML, Kracht L, Eggen BJL, Boddeke E (2018) The kaleidoscope of microglial phenotypes. Front Immunol 9:1753. https://doi.org/10.3389/fimmu.2018.01753
Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA et al (2014) Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82:380–397. https://doi.org/10.1016/j.neuron.2014.02.040
Elmore MRP, Hohsfield LA, Kramar EA, Soreq L, Lee RJ, Pham ST et al (2018) Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17:e12832. https://doi.org/10.1111/acel.12832
Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW (2011) Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE 6:e26317. https://doi.org/10.1371/journal.pone.0026317
Fonseca MI, Chu SH, Hernandez MX, Fang MJ, Modarresi L, Selvan P et al (2017) Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation 14:48. https://doi.org/10.1186/s12974-017-0814-9
Franklin RJM, Ffrench-Constant C (2017) Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci 18:753–769. https://doi.org/10.1038/nrn.2017.136
Garcia JA, Cardona SM, Cardona AE (2013) Analyses of microglia effector function using CX3CR1-GFP knock-in mice. Methods Mol Biol 1041:307–317. https://doi.org/10.1007/978-1-62703-520-0_27
Garcia LM, Hacker JL, Sase S, Adang L, Almad A (2020) Glial cells in the driver seat of leukodystrophy pathogenesis. Neurobiol Dis 146:105087. https://doi.org/10.1016/j.nbd.2020.105087
Gelfand JM, Cree BAC, Hauser SL (2017) Ocrelizumab and Other CD20(+) B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 14:835–841. https://doi.org/10.1007/s13311-017-0557-4
Gerrits E, Brouwer N, Kooistra SM, Woodbury ME, Vermeiren Y, Lambourne M et al (2021) Distinct amyloid-beta and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol 141:681–696. https://doi.org/10.1007/s00401-021-02263-w
Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN et al (2019) Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176(43–55):e13. https://doi.org/10.1016/j.cell.2018.10.049
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. https://doi.org/10.1126/science.1194637
Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S et al (2013) A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 16:1618–1626. https://doi.org/10.1038/nn.3531
Goldmann T, Zeller N, Raasch J, Kierdorf K, Frenzel K, Ketscher L et al (2015) USP 18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J 34:1612–1629
Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E et al (2017) An environment-dependent transcriptional network specifies human microglia identity. Science. https://doi.org/10.1126/science.aal3222
Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP et al (2016) Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci 19:504–516. https://doi.org/10.1038/nn.4222
Green KN, Crapser JD, Hohsfield LA (2020) To Kill a Microglia: A Case for CSF1R Inhibitors. Trends Immunol 41:771–784. https://doi.org/10.1016/j.it.2020.07.001
Greenfield AL, Hauser SL (2018) B-cell therapy for multiple sclerosis: entering an era. Ann Neurol 83:13–26. https://doi.org/10.1002/ana.25119
Gudi V, Moharregh-Khiabani D, Skripuletz T, Koutsoudaki PN, Kotsiari A, Skuljec J et al (2009) Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res 1283:127–138. https://doi.org/10.1016/j.brainres.2009.06.005
Gyoneva S, Hosur R, Gosselin D, Zhang B, Ouyang Z, Cotleur AC et al (2019) Cx3cr1-deficient microglia exhibit a premature aging transcriptome. Life Sci Alliance. https://doi.org/10.26508/lsa.201900453
Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER et al (2017) Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol 134:441–458. https://doi.org/10.1007/s00401-017-1747-1
Hakola HP, Jarvi OH, Sourander P (1970) Osteodysplasia polycystica hereditaria combined with sclerosing leucoencephalopathy, a new entity of the dementia praesenilis group. Acta Neurol Scand 46:79–80
Hakola HPA (1972) Neuropsychiatric and genetic aspects of a new hereditary disease characterized by progressive dementia and lipomembranous polycytic osteodysplasia. Acta Psychiatr Scand Suppl 232:1–173
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A et al (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50(253–271):e256. https://doi.org/10.1016/j.immuni.2018.11.004
Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK et al (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 95:10896–10901. https://doi.org/10.1073/pnas.95.18.10896
Hasel P, Rose IVL, Sadick JS, Kim RD, Liddelow SA (2021) Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci 24:1475–1487. https://doi.org/10.1038/s41593-021-00905-6
Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB et al (2020) Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci 40:2960–2974. https://doi.org/10.1523/JNEUROSCI.2402-19.2020
Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E (2000) Impact of white matter changes on clinical manifestation of Alzheimer’s disease: a quantitative study. Stroke 31:2182–2188. https://doi.org/10.1161/01.str.31.9.2182
Jacobs AJ, Castillo-Ruiz A, Cisternas CD, Forger NG (2019) Microglial depletion causes region-specific changes to developmental neuronal cell death in the mouse brain. Dev Neurobiol 79:769–779. https://doi.org/10.1002/dneu.22706
Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H et al (2019) Lipid-associated macrophages control metabolic homeostasis in a trem2-dependent manner. Cell 178(686–698):e614. https://doi.org/10.1016/j.cell.2019.05.054
Jang H, Kwon H, Yang JJ, Hong J, Kim Y, Kim KW et al (2017) Correlations between gray matter and white matter degeneration in pure alzheimer’s disease, pure subcortical vascular dementia, and mixed dementia. Sci Rep 7:9541. https://doi.org/10.1038/s41598-017-10074-x
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S et al (2019) Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 51:404–413. https://doi.org/10.1038/s41588-018-0311-9
Jensen SK, Michaels NJ, Ilyntskyy S, Keough MB, Kovalchuk O, Yong VW (2018) Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1alpha. Cell Rep 24:3167–3179. https://doi.org/10.1016/j.celrep.2018.08.060
Jordao MJC, Sankowski R, Brendecke SM, Sagar LG, Tai YH, Tay TL et al (2019) Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. https://doi.org/10.1126/science.aat7554
Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A et al (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20:4106–4114. https://doi.org/10.1128/MCB.20.11.4106-4114.2000
Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci 14:198. https://doi.org/10.3389/fncel.2020.00198
Kaiser T, Feng G (2019) Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro. https://doi.org/10.1523/ENEURO.0448-18.2019
Kang SS, Ebbert MTW, Baker KE, Cook C, Wang X, Sens JP et al (2018) Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J Exp Med 215:2235–2245. https://doi.org/10.1084/jem.20180653
Kempthorne L, Yoon H, Madore C, Smith S, Wszolek ZK, Rademakers R et al (2020) Loss of homeostatic microglial phenotype in CSF1R-related Leukoencephalopathy. Acta Neuropathol Commun 8:72. https://doi.org/10.1186/s40478-020-00947-0
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK et al (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169(1276–1290):e1217. https://doi.org/10.1016/j.cell.2017.05.018
Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG et al (2013) Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16:273–280. https://doi.org/10.1038/nn.3318
Kim CC, Nakamura MC, Hsieh CL (2016) Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation 13:117. https://doi.org/10.1186/s12974-016-0581-z
Kondo Y, Duncan ID (2009) Selective reduction in microglia density and function in the white matter of colony-stimulating factor-1-deficient mice. J Neurosci Res 87:2686–2695. https://doi.org/10.1002/jnr.22096
Kracht L, Borggrewe M, Eskandar S, Brouwer N, de Sousa C, Lopes SM et al (2020) Human fetal microglia acquire homeostatic immune-sensing properties early in development. Science 369:530–537. https://doi.org/10.1126/science.aba5906
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712. https://doi.org/10.1093/brain/awh641
Lall D, Lorenzini I, Mota TA, Bell S, Mahan TE, Ulrich JD et al (2021) C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation. Neuron 109(2275–2291):e2278. https://doi.org/10.1016/j.neuron.2021.05.020
Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170. https://doi.org/10.1016/0306-4522(90)90229-w
Levit A, Regis AM, Gibson A, Hough OH, Maheshwari S, Agca Y et al (2019) Impaired behavioural flexibility related to white matter microgliosis in the TgAPP21 rat model of Alzheimer disease. Brain Behav Immun 80:25–34. https://doi.org/10.1016/j.bbi.2019.02.013
Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J et al (2019) Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101(207–223):e210. https://doi.org/10.1016/j.neuron.2018.12.006
Li R, Patterson KR, Bar-Or A (2018) Reassessing B cell contributions in multiple sclerosis. Nat Immunol 19:696–707. https://doi.org/10.1038/s41590-018-0135-x
Lloyd AF, Davies CL, Holloway RK, Labrak Y, Ireland G, Carradori D et al (2019) Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nature Neurosci 22:1046. https://doi.org/10.1038/s41593-019-0418-Z
Lloyd AF, Miron VE (2019) The pro-remyelination properties of microglia in the central nervous system. Nat Rev Neurol 15:447–458. https://doi.org/10.1038/s41582-019-0184-2
Maniatis S, Aijo T, Vickovic S, Braine C, Kang K, Mollbrink A et al (2019) Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364:89–93. https://doi.org/10.1126/science.aav9776
Marteyn A, Baron-Van Evercooren A (2016) Is involvement of inflammation underestimated in Pelizaeus-Merzbacher disease? J Neurosci Res 94:1572–1578. https://doi.org/10.1002/jnr.23931
Martin E, El-Behi M, Fontaine B, Delarasse C (2017) Analysis of microglia and monocyte-derived macrophages from the central nervous system by flow cytometry. J Vis Exp 2017:1–7. https://doi.org/10.3791/55781
Masuda T, Amann L, Sankowski R, Staszewski O et al (2020) Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol 21:802–815. https://doi.org/10.1038/s41590-020-0707-4
Masuda T, Sankowski R, Staszewski O, Bottcher C, Amann L, Sagar SC et al (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566:388–392. https://doi.org/10.1038/s41586-019-0924-x
Masuda T, Sankowski R, Staszewski O, Prinz M (2020) Microglia heterogeneity in the single-cell era. Cell Rep 30:1271–1281. https://doi.org/10.1016/j.celrep.2020.01.010
Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S et al (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science. https://doi.org/10.1126/science.aad8670
Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ et al (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570:332–337. https://doi.org/10.1038/s41586-019-1195-2
McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K et al (2014) Motor skill learning requires active central myelination. Science 346:318–322. https://doi.org/10.1126/science.1254960
McKinsey GL, Lizama CO, Keown-Lang AE, Niu A, Santander N, Larpthaveesarp A et al (2020) A new genetic strategy for targeting microglia in development and disease. Elife 9:e54590. https://doi.org/10.7554/eLife.54590
Mecca C, Giambanco I, Donato R, Arcuri C (2018) Microglia and aging: the role of the TREM2-DAP12 and CX3CL1-CX3CR1 axes. Int J Mol Sci. https://doi.org/10.3390/ijms19010318
Meuwissen ME, Schot R, Buta S, Oudesluijs G, Tinschert S, Speer SD et al (2016) Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J Exp Med 213:1163–1174. https://doi.org/10.1084/jem.20151529
Miedema A, Gerrits E, Brouwer N, Jiang Q, Kracht L, Meijer M et al (2021) Brain macrophages acquire distinct transcriptomes prior to demyelination in multiple sclerosis. bioRxiv. https://doi.org/10.1101/2021.10.27.465877
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL et al (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218. https://doi.org/10.1038/nn.3469
Miron VE, Priller J (2020) Investigating microglia in health and disease: challenges and opportunities. Trends Immunol 41:785–793. https://doi.org/10.1016/j.it.2020.07.002
Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP et al (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. https://doi.org/10.1016/j.immuni.2018.01.011
Nasu T, Tsukahara Y, Terayama K (1973) A lipid metabolic disease—“membranous lipodystrophy”—an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Pathol Int 23:539–558
Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nunez V, Johnson KR et al (2015) Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 138:3581–3597. https://doi.org/10.1093/brain/awv289
Oda M (1983) Familial sudanophilic leukodystrophy with multiple and semisystematic spongy foci: Autopsy report of three adult females. International Symposium on the Leukodystrophy and Allied Diseases. Neuropathology: 173–185
Olah M, Patrick E, Villani AC, Xu J, White CC, Ryan KJ et al (2018) A transcriptomic atlas of aged human microglia. Nat Commun 9:539. https://doi.org/10.1038/s41467-018-02926-5
Oosterhof N, Kuil LE, van der Linde HC, Burm SM, Berdowski W, Ijcken WFJ et al (2018) Colony-stimulating factor 1 receptor (CSF1R) regulates microglia density and distribution, but not microglia differentiation in vivo. Cell Rep 24(1203–1217):e1206. https://doi.org/10.1016/j.celrep.2018.06.113
Oyanagi K, Kinoshita M, Suzuki-Kouyama E, Inoue T, Nakahara A, Tokiwai M et al (2017) Adult onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) and Nasu-Hakola disease: lesion staging and dynamic changes of axons and microglial subsets. Brain Pathol 27:748–769. https://doi.org/10.1111/bpa.12443
Paloneva J, Autti T, Raininko R, Partanen J, Salonen O, Puranen M et al (2001) CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology 56:1552–1558. https://doi.org/10.1212/wnl.56.11.1552
Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R et al (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 71:656–662. https://doi.org/10.1086/342259
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ et al (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. https://doi.org/10.1016/j.cell.2013.11.030
Peferoen LA, Vogel DY, Ummenthum K, Breur M, Heijnen PD, Gerritsen WH et al (2015) Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol 74:48–63. https://doi.org/10.1097/NEN.0000000000000149
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400. https://doi.org/10.1002/ana.1123
Petrik MS, Wilson JM, Grant SC, Blackband SJ, Tabata RC, Shan X et al (2007) Magnetic resonance microscopy and immunohistochemistry of the CNS of the mutant SOD murine model of ALS reveals widespread neural deficits. Neuromolecular Med 9:216–229. https://doi.org/10.1007/s12017-007-8002-1
Petrova N, Nutma E, Carassiti D, Rs Newman J, Amor S, Altmann DR et al (2020) Synaptic loss in multiple sclerosis spinal cord. Ann Neurol 88:619–625. https://doi.org/10.1002/ana.25835
Plemel JR, Stratton JA, Michaels NJ, Rawji KS, Zhang E, Sinha S et al (2020) Microglia response following acute demyelination is heterogeneous and limits infiltrating macrophage dispersion. Sci Adv. https://doi.org/10.1126/sciadv.aay6324
Raj D, Yin Z, Breur M, Doorduin J, Holtman IR, Olah M et al (2017) Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front Mol Neurosci 10:206. https://doi.org/10.3389/fnmol.2017.00206
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19:987–991. https://doi.org/10.1038/nn.4338
Rawji KS, Young AMH, Ghosh T, Michaels NJ, Mirzaei R, Kappen J et al (2020) Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol 139:893–909. https://doi.org/10.1007/s00401-020-02129-7
Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H et al (2021) White matter aging drives microglial diversity. Neuron 109(1100–1117):e1110. https://doi.org/10.1016/j.neuron.2021.01.027
Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I et al (2019) The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to abeta plaques. Cell Rep 27(1293–1306):e1296. https://doi.org/10.1016/j.celrep.2019.03.099
Sankowski R, Bottcher C, Masuda T, Geirsdottir L, Sagar SE, Seredenina T et al (2019) Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci 22:2098–2110. https://doi.org/10.1038/s41593-019-0532-y
Sariol A, Mackin S, Allred MG, Ma C, Zhou Y, Zhang Q (2020) Microglia depletion exacerbates demyelination and impairs remyelination in a neurotropic coronavirus infection. Proc Natl Acad Sci U S A 117:24464–24474. https://doi.org/10.1073/pnas.2007814117
Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D et al (2019) Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573:75–82. https://doi.org/10.1038/s41586-019-1404-z
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90. https://doi.org/10.1126/science.1219179
Schwabenland M, Mossad O, Peres AG, Kessler F, Maron FJM, Harsan LA et al (2019) Loss of USP18 in microglia induces white matter pathology. Acta Neuropathol Commun 7:106. https://doi.org/10.1186/s40478-019-0757-8
See P, Lum J, Chen J, Ginhoux F (2018) A single-cell sequencing guide for immunologists. Front Immunol 9:2425. https://doi.org/10.3389/fimmu.2018.02425
Sierksma A, Lu A, Mancuso R, Fattorelli N, Thrupp N, Salta E et al (2020) Novel Alzheimer risk genes determine the microglia response to amyloid-beta but not to TAU pathology. EMBO Mol Med 12:e10606. https://doi.org/10.15252/emmm.201910606
Singh S, Metz I, Amor S, van der Valk P, Stadelmann C, Bruck W (2013) Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 125:595–608. https://doi.org/10.1007/s00401-013-1082-0
Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, MacLean AG et al (2014) Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy. Am J Pathol 184:382–396. https://doi.org/10.1016/j.ajpath.2013.10.011
Stahl PL, Salmen F, Vickovic S, Lundmark A, Navarro JF, Magnusson J et al (2016) Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353:78–82. https://doi.org/10.1126/science.aaf2403
Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC et al (2020) Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105(150–164):e156. https://doi.org/10.1016/j.neuron.2019.10.013
Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L et al (2013) A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci 33:13460–13474. https://doi.org/10.1523/JNEUROSCI.1333-13.2013
Stout JC, Jernigan TL, Archibald SL, Salmon DP (1996) Association of dementia severity with cortical gray matter and abnormal white matter volumes in dementia of the Alzheimer type. Arch Neurol 53:742–749. https://doi.org/10.1001/archneur.1996.00550080056013
Svensson V, Nath K, Natarajan L-H, Miragaia RJ, Labalette C, Macaulay IC (2017) Power analysis of single cell RNA-sequencing experiments Europe PMC Funders Group. Nat Methods 14:381–387. https://doi.org/10.1038/nmeth.4220.Power
Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L et al (2016) Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 7:11499. https://doi.org/10.1038/ncomms11499
Tada M, Konno T, Tada M, Tezuka T, Miura T, Mezaki N et al (2016) Characteristic microglial features in patients with hereditary diffuse leukoencephalopathy with spheroids. Ann Neurol 80:554–565. https://doi.org/10.1002/ana.24754
Takata K, Ginhoux F (2015) Poised for action: USP18 restrains microglial activation in the white matter. EMBO J 34:1603–1605. https://doi.org/10.15252/embj.201591899
Tanaka J (2000) Nasu-Hakola disease: a review of its leukoencephalopathic and membranolipodystrophic features. Neuropathology 20(Suppl):S25-29. https://doi.org/10.1046/j.1440-1789.2000.00297.x
Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar DM et al (2017) A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20:793–803. https://doi.org/10.1038/nn.4547
Thomas WE (1990) Characterization of the dynamic nature of microglial cells. Brain Res Bull 25:351–354. https://doi.org/10.1016/0361-9230(90)90083-c
Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B et al (2014) Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 261:1–16. https://doi.org/10.1007/s00415-013-7169-7
van der Knaap MS, Bugiani M (2017) Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol 134:351–382. https://doi.org/10.1007/s00401-017-1739-1
van der Poel M, Ulas T, Mizee MR, Hsiao CC, Miedema SSM, Adelia SKG et al (2019) Transcriptional profiling of human microglia reveals grey-white matter heterogeneity and multiple sclerosis-associated changes. Nat Commun 10:1139. https://doi.org/10.1038/s41467-019-08976-7
van Horssen J, Singh S, van der Pol S, Kipp M, Lim JL, Peferoen L et al (2012) Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J Neuroinflammation 9:1–9
Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, Prijck S et al (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22:1021–1035. https://doi.org/10.1038/s41593-019-0393-4
van Wageningen TA, Antonovaite N, Paardekam E, Breve JJP, Iannuzzi D, van Dam AM (2021) Viscoelastic properties of white and gray matter-derived microglia differentiate upon treatment with lipopolysaccharide but not upon treatment with myelin. J Neuroinflammation 18:83. https://doi.org/10.1186/s12974-021-02134-x
van Wageningen TA, Vlaar E, Kooij G, Jongenelen CAM, Geurts JJG, van Dam AM (2019) Regulation of microglial TMEM119 and P2RY12 immunoreactivity in multiple sclerosis white and grey matter lesions is dependent on their inflammatory environment. Acta Neuropathol Commun 7:206. https://doi.org/10.1186/s40478-019-0850-z
Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P et al (2013) Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 10:1–12
von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol 524:3865–3895. https://doi.org/10.1002/cne.24040
Wang F, Ren SY, Chen JF, Liu K, Li RX, Li ZF et al (2020) Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat Neurosci 23:481–486. https://doi.org/10.1038/s41593-020-0588-8
Werneburg S, Jung J, Kunjamma RB, Ha SK, Luciano NJ, Willis CM et al (2020) Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52(167–182):e167. https://doi.org/10.1016/j.immuni.2019.12.004
White GE, Greaves DR (2012) Fractalkine: a survivor’s guide: chemokines as antiapoptotic mediators. Arterioscler Thromb Vasc Biol 32:589–594. https://doi.org/10.1161/ATVBAHA.111.237412
Willis EF, MacDonald KPA, Nguyen QH, Garrido AL, Gillespie ER, Harley SBR et al (2020) Repopulating microglia promote brain repair in an il-6-dependent manner. Cell 180(833–846):e816. https://doi.org/10.1016/j.cell.2020.02.013
Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R et al (2017) A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J 36:3292–3308. https://doi.org/10.15252/embj.201696056
Wlodarczyk A, Khorooshi R, Marczynska J, Holtman IR, Burton M, Jensen KN et al (2021) Type I interferon-activated microglia are critical for neuromyelitis optica pathology. Glia 69:943–953. https://doi.org/10.1002/glia.23938
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496:445–455. https://doi.org/10.1038/nature12034
Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD et al (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19:1210–1217. https://doi.org/10.1038/nn.4351
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R et al (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211:1533–1549. https://doi.org/10.1084/jem.20132477
Yin J, Valin KL, Dixon ML, Leavenworth JW (2017) The role of microglia and macrophages in CNS homeostasis, autoimmunity, and cancer. J Immunol Res 2017:5150678. https://doi.org/10.1155/2017/5150678
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M et al (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91. https://doi.org/10.1016/j.immuni.2012.12.001
Zhou T, Ahmad TK, Gozda K, Truong J, Kong J, Namaka M (2017) Implications of white matter damage in amyotrophic lateral sclerosis (Review). Mol Med Rep 16:4379–4392. https://doi.org/10.3892/mmr.2017.7186
Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H (2017) Loss of “homeostatic” microglia and patterns of their activation in active multiple sclerosis. Brain 140:1900–1913. https://doi.org/10.1093/brain/awx113
Zrzavy T, Machado-Santos J, Christine S, Baumgartner C, Weiner HL, Butovsky O et al (2018) Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol 28:791–805. https://doi.org/10.1111/bpa.12583
Acknowledgements
We thank Prof. Dr. M.S. van der Knaap, Prof. Dr. N. Wolf., and Dr. M. Bugiani for supplying tissue from ALSP and MLD for the pathology figure.
Author information
Authors and Affiliations
Contributions
S.A., N.B.M., E.G., M.M., S.K., V.E.M. and E.N. researched data for the article, and wrote the manuscript. All authors reviewed, edited and agreed on the content of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amor, S., McNamara, N.B., Gerrits, E. et al. White matter microglia heterogeneity in the CNS. Acta Neuropathol 143, 125–141 (2022). https://doi.org/10.1007/s00401-021-02389-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02389-x