Abstract
Biologics — medications derived from a biological source — are increasingly used as pharmaceuticals, for example, as vaccines. Biologics are usually produced in bacterial, mammalian or insect cells. Alternatively, plant molecular farming, that is, the manufacture of biologics in plant cells, transgenic plants and algae, offers a cheaper and easily adaptable strategy for the production of biologics, in particular, in low-resource settings. In this Review, we discuss current vaccination challenges, such as cold chain requirements, and highlight how plant molecular farming in combination with advanced materials can be applied to address these challenges. The production of plant viruses and virus-based nanotechnologies in plants enables low-cost and regional fabrication of thermostable vaccines. We also highlight key new vaccine delivery technologies, including microneedle patches and material platforms for intranasal and oral delivery. Finally, we provide an outlook of future possibilities for plant molecular farming of next-generation vaccines and biologics.
Similar content being viewed by others
Introduction
A human analogue of insulin (Humulin) was the first biologic introduced into the market in 1983. Since then, biologics have been increasingly used as pharmaceutical agents, outpacing the market of small-molecule drugs1 with an estimated market size of around US$400 billion by 2025 (ref.2). Biologics are a class of medications that are derived from a biological source and typically fall into one of four major categories: monoclonal antibodies (mAbs), receptor modulators, enzyme modulators and vaccines3. Vaccines have been crucial in eliminating infectious diseases, such as smallpox4 and rinderpest in 2011 (ref.5), and in eradicating once-deadly diseases, such as diphtheria, measles, polio and rubella, by 99%, with many other diseases, such as mumps, pertussis and tetanus, nearing that stage. In the USA alone, it is estimated that in just one generation of children, 13 different vaccines have prevented up to 20 million diseases and 40,000 deaths, with economic savings of up to US$69 billion4. On a global scale, these numbers are much higher; one estimate shows that ten vaccines deployed in 94 low- and middle-income countries led to a US$586 billion reduction in illness-related costs, and a reduction of up to US$1.53 trillion when considering additional economic benefits6.
Vaccines are usually manufactured using cell-based expression systems, such as mammalian and insect cell lines, bacterial or yeast cultures. Plant molecular farming, that is, using plant cells or plants as expression platforms, is a rapidly emerging alternative, which was originally introduced in 1986 for the production of human growth hormone (HGH) in transgenic tobacco and sunflowers7. Plant molecular farming is currently used for the production of seasonal influenza vaccines8 and for Elelyso for Gaucher disease in the USA9. Although plant molecular farming may not replace industrial expression systems, it may have a unique role in the generation of vaccines in low-resource areas, for targeting niche and orphan vaccines, and for the production of virus nanoparticles (VNPs) for vaccine applications10. Furthermore, the rise of the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has focused research attention on novel approaches to vaccines. Traditional vaccines are either live attenuated (that is, less virulent forms of the original pathogen), inactivated (that is, inactivated pathogen without disease-producing capacity), or recombinant proteins and viral vectors. In addition, the two vaccines that were first granted emergency authorization in the USA for COVID-19 are messenger RNA (mRNA) vaccines delivered in lipid nanoparticles11.
In this Review, we discuss challenges in current vaccine development and distribution, such as cold chain disruptions and lack of health-care professionals, which are particularly problematic in low-resource areas. Cold chain requirements pose a general logistical challenge for vaccines, as highlighted by the current rollout of the mRNA-based COVID-19 vaccines12, and traditional administration techniques, such as injections, require trained health-care professionals. Here, we investigate how molecular farming and nanotechnology-based strategies could provide an alternative strategy to traditional vaccines, enabling rapid development, effective deployment and safe administration of vaccines.
Vaccine technology
A brief history of vaccines
The history of modern vaccination began with Edward Jenner’s ‘live attenuated’ smallpox vaccine in the late eighteenth century13. Nearly a century later, in 1885, Louis Pasteur developed a rabies vaccine by drying the brains of infected rabbits; however, this vaccine was rather unpredictable and often caused serious side effects14. Shortly thereafter it was found that heat or chemical treatment could be used to inactivate bacteria, and vaccines for typhoid (Salmonella typhi), plague (Yersinia pestis) and cholera (Vibrio cholerae) were developed13. Vaccine production was greatly improved in the 1950s, when the development of cell culture techniques enabled the in vitro production of non-virulent viruses instead of requiring to be isolated from infected animals15. Bacterially expressed hepatitis B surface antigen then became the first vaccine produced by recombinant DNA technology16, which laid the foundation for the four major categories of modern vaccines: live attenuated, killed/inactivated whole organisms, subunit and toxoid vaccines (Table 1). Since then, new vaccine types have emerged, such as viral vectors and nucleic acid vaccines17. Today, 85 human vaccines and vaccine combinations are on the market.
Challenges and innovations
Materials design can greatly improve vaccination approaches by enabling stabilization and controlled release, and/or by providing delivery devices for self-administration, which is particularly important for vaccines against viruses with a great degree of variability, such as the human immunodeficiency viruses (HIV-1 and HIV-2). HIV-1 shows worldwide variability as well as direct infection and destruction of immune cells. In addition, there is no validated animal model available for HIV-1 research, and the high rate of mutation allows the virus to escape antibody responses to targeted antigens18. High variability may be addressed by universal vaccines, which are effective against all virus subtypes and are impervious to future mutations by the pathogen. For example, the Gardasil-9 vaccine provides immunity against nine human papillomavirus (HPV) types, which should prevent >90% of HPV infections and subsequent cervical cancer19; however, expanding immunity protection to all known HPVs would enable HPV eradication. A universal vaccine against influenza would also greatly decrease infection rates, health-care costs and associated mortality, while improving patient compliance20. Nanotechnology-based approaches, such as HPV-encapsidating microneedles21 and slow-release implant technologies22, could further improve immunization against these difficult pathogens.
Vaccines are also being developed for non-infectious pathophysiological conditions, such as autoimmune diseases and cancers23. These vaccines are difficult to produce, because the molecular causes and antigens are often specific to the individual patient. Therefore, unlike for infectious disease vaccines, here it may be more advantageous to produce patient-specific vaccines — a considerable challenge for conventional production methods. Improvements in biotechnology methods, such as whole-exome or RNA sequencing24, may allow the isolation of patient-specific antigens for the creation of specialized vaccines.
Nanotechnology-based approaches have also played a key part in the development of COVID-19 mRNA vaccines, although it remains to be seen whether mRNA vaccines will provide long- or short-lasting immunity against SARS-CoV-2. Furthermore, the lack of thermostability of lipid nanoparticle formulations requiring storage and distribution in ultralow-temperature freezers presents a great challenge for vaccine distribution to rural and low-resource regions because these vaccines are temperature-stable only when frozen at –20 to –80 °C. Viral vector vaccines may be better suited for vaccination in remote areas, because they can be stored at higher temperatures of up to 4 °C. However, early reports suggest that viral vector vaccines do not reduce symptomatic infection as well as mRNA vaccines25, although the differing trial protocols and demographics make it difficult to compare the trials equally.
Traditional biologics expression
Humulin, the first biologic used in humans1, is expressed in Escherichia coli26. Advances in DNA technology have further enabled vaccine production in yeast, for example, for the production of subunit vaccines against the hepatitis B virus (HBV)27. In addition, mammalian cells, insect cells and chicken eggs can be applied as expression systems for vaccine production.
Bacteria are the most widely used expression system, benefiting from low cost, rapid growth and ease of use; in ideal conditions, E. coli can double its biomass within 20 minutes28. An estimated 30% of all biopharmaceuticals are currently engineered in bacterial systems, with E. coli being the most widely used strain29. Bacterial systems are also applied to generate DNA plasmids, which are subsequently used to produce the final biologic. The main disadvantage of bacterial systems is the lack of eukaryotic post-translational modifications. Without proper post-translational modification, purified proteins may behave differently in vivo26 compared with their non-recombinant counterparts, which can lead to diminished or total loss of activity, reduced half-life and decreased stability and/or immunogenicity30. Moreover, the presence of rare codons in eukaryotic genes can be problematic, because they can cause early termination during bacterial protein production, necessitating the redesign of genes31.
To overcome the problems associated with bacterial protein production, mammalian cell lines can be used. From 2016 to 2018, 84% of pharmaceutical proteins were made in mammalian cell lines, predominantly in Chinese hamster ovary (CHO) cells32. CHO cells achieve high protein yield (up to 10 g l–1 for some proteins)33, they can grow in suspension34 and they can withstand changes in external factors, such as temperature and pH35. However, although post-translational modifications in CHO cells more closely resemble those of human cells, they are not identical and can induce immune or other adverse reactions in patients36.
Alternatively, insect cells replicate faster than mammalian cells, enabling faster protein expression, but are more costly than bacteria owing to the requirement of specialized culture media28. A main disadvantage of insect cells is also the difference in post-translational modifications, as compared with human cells, which can make the biologics immunogenic37. Furthermore, some insects carry pathogenic viruses (for example, arboviruses) and, thus, insect cell lines must be closely examined before regulatory approval38. Furthermore, insect cells can produce proteases in response to viral transfection that can digest the protein of interest37.
Finally, chicken eggs are widely used for vaccine production, for example, for influenza vaccines39. Although well established, antigenic drift in chicken eggs can decrease vaccine efficacy, compared with vaccines produced in cell-based systems40. In addition, vaccine production in chicken eggs can take up to 6 months, whereas insect cell systems can produce vaccines in 6–8 weeks39. Possible contamination with human pathogens can also be a problem, which can be avoided by molecular farming41.
Molecular farming
Since the production of HGH in transgenic tobacco7, many proof-of-concept and efficacy tests have been performed of plant-made therapeutics and vaccines for humans and animals42 (Table 2). A tobacco-produced mAb used in the production of a hepatitis B subunit vaccine43 and a Newcastle disease subunit vaccine for poultry made in cultured tobacco cells were the first plant-made recombinant proteins to receive regulatory approval in 2006 (ref.44) (Fig. 1). However, only one human therapeutic (Elelyso, a mitochondrial enzyme deficit therapy for Gaucher disease) produced by molecular farming has thus far been licensed by the US Food and Drug Administration (FDA)9. In addition, the first phase III human clinical trial for a plant-produced vaccine has just been successfully concluded. This virus like particle (VLP)-based quadrivalent seasonal influenza virus vaccine is currently undergoing final consideration for licensure in Canada8. The fact that only a few plant-produced therapeutic products have been clinically translated thus far may be more related to industrial and regulatory inertia than to product inadequacy, especially given the plentiful evidence of functional equivalency.
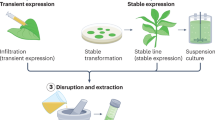
Transgenic plant and plant cell production of proteins laid the foundation of plant molecular farming; however, it was the advent of transient expression technologies that unleashed its true potential, including plant virus-derived vectors, somatic transformation of cells in normal plants (usually by infiltration of Nicotiana benthamiana with Agrobacterium suspensions), and hybrid methods involving delivery of replicating vectors by Agrobacterium42 (Box 1). These transient technologies enable protein expression within days of gene cloning, instead of relying on the generation of stable transgenic plants, which can take years. Moreover, yields are usually higher and more consistent, than transgenic plant systems, because these techniques do not depend on genome insertion sites and expression is not downregulated by methylation or other gene modifications, enabling much faster experimentation and optimization of expression. In addition, production scale-up is more efficient than transgenic plants and conventional cell-based technologies. Only a small volume of recombinant Agrobacterium suspension has to be grown for the biologic of interest, which can then be combined by agroinfiltration with as many plants — grown cheaply in nearby facilities — as required. It is striking that Medicago Inc. received funding from the US Defense Advanced Research Projects Agency (DARPA) Blue Angel initiative to produce ten million doses of current good manufacturing practice (cGMP)-level influenza vaccines 1 month after receiving the sequence of the virus45. Medicago Inc. has further announced preliminary success in investigating a VLP-based COVID-19 vaccine candidate, which was produced in just over 20 days after receiving the spike protein gene sequence46. Phase I clinical trial results showed ten times more production of neutralizing antibodies than in convalescent sera47. This vaccine candidate has recently entered phase II/III clinical trials and is administered along with GlaxoSmithKline’s AS03 adjuvant48.
Algal cell expression
The microalga Chlamydomonas reinhardtii is the most commonly used algal system for molecular farming49 and has been applied to express mAbs (against glycoprotein D of the herpes simplex virus and human fibronectin type III), subunit vaccines (viral protein (VP) 1 of foot-and-mouth disease), allergens (peanut allergens), growth factors (vascular endothelial growth factor), and immunotoxins (chimeric antibody to CD22 and exotoxin A)50. Although the genomes of the mitochondria, nucleus and chloroplast of C. reinhardtii have been sequenced51, expression has been mainly applied in chloroplasts thus far, because the chloroplast genome is small and simple50, and biologics can be sequestered in the chloroplast without harming the host cells or being degraded51. However, similar to bacterial expression, post-translational modifications are lacking in this system. Alternatively, Lemna and moss systems have been explored for biologic expression52,53.
Plants and plant cells
Using plants for vaccine production has the advantage that plants are cheaper than mammalian systems in terms of biomass production, and they have similar protein folding, assembly and glycosylation54. Therefore, plants can be applied to efficiently produce a range of pharmaceuticals at low cost. It is estimated that the final cost of producing biologics in plants is 68% of that in conventional production systems (site and batch costs are lower in plants, whereas downstream processing costs are identical)55. Furthermore, post-translational modifications, such as glycosylation, can be manipulated in plants. Thus, plant-produced products have appropriate glycan structures, which can improve half-lives of products and ease downstream processing56. For example, Elelyso is considered a ‘biobetter’ (biologics that improve upon existing biologics), compared with its CHO-produced counterpart, because the end product contains terminal mannose residues that aid in macrophage receptor binding57. In CHO cell production, mannose residues are attached in vitro, adding an extra step and increasing the costs58. Influenza virus haemagglutinin proteins made in plants were shown not to induce problematic adverse effects in clinical trials despite non-mammalian glycosylation59. In proteins that require human glycosylation, such as the HIV-1 Env gp140 protein, the yield can be substantially increased and the glycosylation pattern of HIV-1 Env gp140 can be altered through coexpression of human chaperonins in N. benthamiana plants, engineered to not produce xylose or fucose transferases60.
Plants and plant cells are also inherently safer than bacterial and mammalian systems owing to the low possibility of harmful contamination. In contrast to these systems, plants do not produce endotoxins and cannot be infected by pathogens harmful to humans61. Therefore, plants could potentially be used in edible vaccines62, although concerns of cross-contamination and accidental vaccination of wildlife have stalled development in this field.
Various vaccine candidates have already been produced by molecular farming, including HIV-1 Env60, ten different HPV L1 proteins63, West Nile virus envelope protein64 and dengue virus VLPs65. Notably, this list includes three different envelope glycoproteins or their derivatives. In addition, a recombinant double-stranded DNA (dsDNA) molecule has been encapsidated in HPV-16 pseudovirions, which were made in plants66, proving that DNA vaccines and their delivery systems can be succesfully produced in plants. Similarly, mRNA vaccines can be produced in plants; for example, recombinant mRNA molecules can be expressed in plants from DNA vectors and specifically encapsidated by adding an assembly signal in tobacco mosaic virus (TMV) coat proteins. Synthetic mRNAs made in vitro can also be encapsidated with purified TMV coat proteins, which was first demonstrated in 1956 with TMV RNA10. Encapsulation of target and self-replicating mRNA can be achieved in plants through co-expression of the desired mRNA and the TMV coat proteins67, making this an attractive and scalable approach towards plant-produced mRNA vaccines.
The limited commercialization of plant cell culture-based expression systems may be related to the lower protein yield (usually 0.01–10 mg l–1, although up to 247 mg l–1 have been reported)68, compared with bacterial and mammalian cell culture, which can achieve up to 10 g l–1 (ref.33). Additionally, controlling the expression levels of certain proteins remains difficult, especially in transgenic plants. Expression can vary between different generations of plants, is highly dependent on the plant type, and can even fluctuate within the same plant’s tissues and organs42. Plants may also suffer from variability of environmental conditions, such as droughts and extreme heat — although this is less problematic in growth houses.
Cookie technique
The cookie technique, that is, the transient transformation of plant cells to produce high-value proteins, may revolutionize the small-scale production of recombinant proteins and other biologics in cultured plant cells69. Here, cell packs or ‘cookies’ are made on porous supports by filtration of suspended cells, followed by incubation with Agrobacterium suspensions without resuspension. The bacteria are then washed out with growth medium, leaving behind a mass with many air spaces. The treatment results in transformation rates of up to 100%, compared with around 1% from co-incubation of cells with Agrobacterium in suspension70. Cell packs can be incubated for days in situ and can produce up to 47 mg kg–1 of recombinant protein. The technique is scalable from microlitre and millilitre volumes up to large-volume preparative columns used for filtration or chromatography, and thus can be used for high-throughput and low-volume screening and for subsequent production of trial batches of proteins.
Clinical applications of molecular farming
Patient-specific antigens
Although molecular farming may not displace fermentation and large-volume cell culture for the production of blockbuster vaccines and therapeutics, it may be advantageous in certain applications; for example, for patient-specific or individualized production of antigens for therapeutic vaccines, which are required for certain diseases, such as non-Hodgkin lymphoma71. mAbs can be produced with relative ease in plants and, thus, non-Hodgkin lymphoma was an early target for molecular farming72. Here, the mAbs are produced by introducing recombinant TMV (magnICON) vectors into N. benthamiana plants using Agrobacterium73. Grams of purified protein, enough for a lifetime supply for a patient, were produced at a cost of only about US$15,000 (2015); for comparison, production by conventional expression systems is estimated to cost around ten times more.
Niche and orphan diseases
Molecular farming also has potential for the production of niche or orphan vaccines as well as therapies, for which the market is perceived as too small to invest in a potential vaccine (for example, Lassa fever in West Africa), and/or the target market cannot afford the costs (for example, Rift Valley fever in East Africa). Importantly, such niche or orphan diseases have a role in the One Health Initiative, which aims to produce reagents for a disease that can infect wildlife, livestock or humans and to repurpose these into vaccines for animals or humans74. For example, the nucleoproteins of both Crimean–Congo haemorrhagic fever75 and Rift Valley fever76 bunyaviruses have been produced in plants with high yield and used in validated serological assays. In addition, SARS-CoV-2 spike glycoproteins have been made in plants in various forms for laboratories and detection kit manufacturers77.
Virus nanoparticles and virus-like particles
Plant virus-derived VNPs are best produced in their natural hosts, that is, plants, by infection or recombinant expression, yielding milligrams of VNPs or VLPs per gram of leaf tissue10. VLPs are the non-infectious version of VNPs and do not contain the viral genome. Plant VNPs are proteinaceous nanomaterials that self-assemble into precise geometries at the nanometre scale. Many of their coat proteins have been mapped to near-atomic resolution, allowing functionalization with spatial control; molecular payloads can be incorporated into the interior cavity, integrated at interfaces, or displayed at the surface using an array of chemical biology approaches78 (Fig. 2a–c). Thus, VNPs can be used as nanocarriers with a variety of payloads, including small-molecule drugs and prodrugs, nucleic acids, therapeutic proteins, contrast agents and photosensitizers for drug delivery79, vaccines80, diagnosis81, theranostics82, catalysis83, live imaging84 and agricultural applications85. Plant-made VNPs confer additional safety benefits compared with their mammalian viral vector counterparts because plant viruses are non-infectious to mammals86. Therefore, this technology is ideal for human vaccines and immunotherapies.
a | Display of epitopes or peptides on cowpea mosaic virus (CPMV) through genetic fusion or bioconjugation. b | Encapsidation of molecular payloads within the cowpea chlorotic mosaic virus (CCMV) through dis-assembly and re-assembly of coat proteins following changes in the solvent conditions. c | Delivery of molecular payloads with tobacco mosaic virus (TMV) using internal glutamate (Glu) or external tyrosine (Tyr) residues. Therapeutic payloads can be loaded into the interior of the TMV or conjugated to the exterior surface of TMV. d | Epitope delivery using plant viral nanoparticles (VNPs). Vaccination with VNPs leads to intracellular processing of the antigen or genomic material encoding the antigen. The antigen is then displayed on the cell surface, leading to the activation of CD4+ and CD8+ T cells. CD4+ T cells go on to activate memory B cells, leading to immune memory against future infections. APC, antigen-presenting cell; BCR, B cell receptor; MHC, major histocompatibility complex; VLP, virus-like particle. The plant VNPs were drawn using UCSF Chimera (CPMV PDB ID: 1NY7; CCMV PDB ID: 1CWP; TMV PDB ID: 2TMV), and parts of Fig. 3d were made using https://smart.servier.com/.
Although non-infectious to mammals, the repetitive, multivalent coat protein assemblies are pathogen-associated molecular patterns (PAMPs) that act as danger signals. VNPs administered via various routes (including subcutaneously and intramascular) drain efficiently to lymph nodes and activate immune cells upon recognition by pattern recognition receptors. VNP-based vaccines also facilitate antigen cross-presentation, which is crucial for major histocompatibility complex class I (MHC-I) presentation of extracellular antigens to trigger a robust cytotoxic T cell response. For example, plant VNPs, including papaya mosaic virus, TMV and potato virus X, can generate robust cellular responses against fused epitopes87. The potency of the immunostimulatory properties of VNPs has been demonstrated in an in situ cowpea mosaic virus (CPMV) vaccine, showing remarkable efficacy in animal models of melanoma, glioma, breast, colon and ovarian cancer88,89,90,91,92. Here, the VNPs are administered directly into the tumour to stimulate innate immune cells within the tumour microenvironment, priming tumour cell killing and antigen processing, which results in systemic antitumour immunity. In contrast to oncolytic viral tumour therapy, pre-existing immunity does not decrease the effectiveness of the immune response induced by the VNPs89. Instead, antibody recognition increases opsonization of CPMV, thereby improving the recognition of the virus by innate immune cells, which are its natural targets. The CPMV cancer immunotherapy has also shown efficacy in companion animals with spontaneous tumours93. Therefore, VNPs are excellent epitope delivery platforms for antigens (Fig. 2d) and adjuvants for vaccine and immunotherapy applications94. The current preclinical development pipeline for VNP-based vaccines includes infectious, cardiovascular and autoimmune diseases as well as substance abuse95. Plant VNP vaccine platform technologies also hold promise for pandemic or epidemic vaccines96 — owing to their high thermal stability they would not be subject to cold chain distribution and/or could be produced in the region for the region through molecular farming.
Distribution challenges of vaccines
The successful vaccination of entire populations in low-resource areas is an arduous task, which will require addressing distribution challenges in relation to cold chain failures and the lack of trained health-care providers with vaccine experience97.
The cold chain
The majority of traditional (live and inactivated) vaccines approved for use are advised to be distributed through the cold chain at 2–8 °C for optimal activity. The cold chain is a temperature-controlled supply chain of the people, equipment and protocols used in the transportation, storage and handling of vaccines from manufacturer to patient (Fig. 3). When disrupted, vaccine denaturation can lead to less effective vaccines with greater side effects. The ‘last mile’ vaccine distribution, describing the allocation of vaccines from national distribution centres to regional facilities and patients, is especially challenging, as evidenced in the SARS-CoV-2 vaccine rollout. The World Health Organization (WHO) reported that in 2011, 2.8 million vaccine doses were lost owing to cold chain disruptions in five countries surveyed. Therefore, in 2012, the WHO expressed a preference for vaccines that are heat- and freeze-stable for extended periods above 8 °C, as a part of the controlled temperature chain98.
Cold chain disruptions can occur from a variety of sources. Vaccines can be exposed to extended periods of heating owing to electricity and power outages, equipment failure, limited ice supplies, and transportation and delivery delays99. Vaccine function can also be compromised by vaccine freezing owing to ice build-up in refrigerators or improper ice conditioning98.
Immunization challenges
Overcoming immunization challenges requires collaboration between multiple parties involved in the vaccine supply chain. First, a better understanding of the impact of heat or freeze exposure on vaccine stability is needed. The WHO further demands that all vaccines purchased by the United Nations Children’s Fund must have vaccine vial monitors100 that measure cumulative heat exposure and signal whether the vaccine should be discarded in case of cold chain disruptions. Vaccine vial monitors are cheap and indispensable; however, they can fail; for example, an oral polio vaccine with a 48-hour controlled temperature chain (indicated by the manufacturer) maintained viable potency for up to 86.9 hours above 8 °C, even though the vaccine vial monitor indicated it had reached its end point101. In addition, it is important that health-care professionals are trained to read and interpret vaccine vial monitor data and in handling procedures, such as preventing sunlight exposure, which can lead to false positives and premature vaccine discards102. Similarly, freeze indicators and shake-tests for freeze-induced protein aggregation can measure potency, in cases when freezing temperatures are a concern98.
Thermostable liquid and dry vaccine formulations can further address cold chain-related challenges. Liquid vaccines can benefit from high-throughput optimization of formulation properties, such as buffer type, pH and ionic strength, to improve stability. Dry vaccines can be more thermostable but must be reconstituted before use. Reconstitution is limited by large product volumes, logistical concerns, and potential sources of error and contaminants. Dry vaccines need simple-to-use reconstitution systems or strategies to bypass the need for reconstitution, for example, by applying aerosols, dry powder jet injection, microneedle patches or biodegradable implants103. Moreover, the ability to withstand temporary storage or transport without refrigeration or ice would help mitigate cold chain disruptions, particularly in areas where health workers travel by foot104 or where there is a lack of cold boxes102.
Health personnel
Health-care workers connect every step of the vaccine supply chain and, thus, require adequate training for each vaccine, including reconstitution methods, handling of the cold chain and controlled-temperature chain, and reading of vaccine vial monitors. Negligent handling may result in wasted vaccines or the delivery of ineffective vaccines103. Therefore, easy-to-use vaccines that require less training may reduce human error, for example, single-use or self-administered systems. UniJect, which is a pre-filled single-use system105, is easy to use, activated by simply pushing the needle through a membrane and into the drug reservoir, and its valve ensures that the vaccine can only be used once by preventing the needle from retracting. The UniJect system has also been reported to be less painful and anxiety-inducing for patients105.
Cost per dose
The definition of successful vaccine implementation could further be redefined from cost per dose to cost per dose delivered, to reflect the true cost of introducing a new vaccine104. A higher cost per dose would probably be accepted for a more stable vaccine, because of reduced wastage, lower storage and handling costs, better efficacy and protection, and easier management, as compared with less-stable vaccines, making it more cost-effective overall. For example, single-use systems, such as UniJect, add $US0.15–0.30 to the cost per dose, compared with traditional single-use syringes; however, the 20% wastage rate for multi-dose vials and the fact that UniJect can be administered at home lead to a decrease in cost per dose delivered, from $US7.19 to $US6.57 in the case of tetanus106. Therefore, dose-sparing technologies in vaccine manufacturing, deployment and administration can have a substantial impact on immunization success and costs.
Plant molecular farming to overcome vaccination challenges
Plants can be used to produce vaccines at small scale and low cost in regions where vaccines are most needed, particularly in areas where it may be difficult to set up cGMP-level bacterial and mammalian cell culture systems. In addition, contaminants from plant molecular farming are not as harmful as endotoxins produced in bacterial expression61. Moving vaccine manufacturing on-site would also reduce cold chain requirements, because long-distance shipping would not be needed; this has been especially highlighted during the SARS-CoV-2 vaccine rollout. In 67 low-income countries, an estimated 90% of the population may not gain access to any SARS-CoV-2 vaccine in 2021 (ref.107). Therefore, plant molecular farming could reduce vaccine inequity and immunization costs108. Furthermore, production of vaccines by plant molecular farming does not require extensive space; for example, it was calculated that Italy could reach a 60% vaccination rate with 12,500 square metres of greenhouses45. Moreover, molecular farming is easily scalable, compared with fermentation systems, which must be adapted and optimized for scale-up, and which require costly bioreactors. By contrast, each plant is a bioreactor, and scale-up is simply achieved by growing more plants.
Plant molecular farming is particularly useful for producing plant viruses as nanocarriers for vaccines. Plant VNPs are very stable and can withstand temperatures outside cold chain requirements; for example, CPMV withstands temperatures of 37 °C for 46 days109 and 60 °C for up to 1 hour110. Thermostability is especially important in areas in which resources for adequate cold chain distribution of vaccines may be limited. Furthermore, plant viruses, owing to their intrinsic stability, can withstand the processing and manufacturing steps of vaccine packaging into new administration technologies, such as microneedles96. VNPs do not only act as carriers, but also as adjuvants, reducing the number of vaccine components. Moreover, the simplicity of VNP-based vaccines can improve health-care worker compliance and reduce the need for training in vaccine administration.
Vaccine administration technologies
Administration of vaccines with hypodermic needles, which is currently the most frequently used administration technology, has several drawbacks. For example, blood-borne pathogens can easily be spread by needle reuse or mishandling111. Moreover, patients may be apprehensive about needles112. Vaccines delivered by hypodermic needles also rely on liquid formulations for injections, which require a cold chain113.
To overcome these obstacles, vaccines can be encapsulated in polymeric materials. Proteins in the solid state embedded within a polymer matrix further improve the thermal stability of the vaccine, thereby decreasing strict adherence to the cold chain114. Polymeric materials could be used in new vaccine delivery platforms that could supplant hypodermic needles, improving patient compliance and reducing the unintended spread of blood-borne pathogens. Next-generation polymeric delivery platforms that are safe, effective and self-administered could thus greatly strengthen concerted vaccination efforts.
Polymeric materials
Considerable research has been devoted to the development of biocompatible and biodegradable polymeric materials for vaccines115 (Table 3). These materials can be of synthetic or natural origin, and processing and manufacturing requirements can differ for each polymer, which may restrict the use of biological components. For example, inactivated or live attenuated vaccines can denature at high temperatures, which restricts the processing conditions (some processes require temperatures above 70 °C) required to encapsulate these vaccines in polymeric materials113. By contrast, VLPs and VNPs are chemo- and thermostable, enabling a broader array of device manufacturing conditions116. Fabricated by plant molecular farming, VLPs and VNPs can be produced at large scales for material integration at lower cost, as compared with conventional inactivated or live attenuated vaccines108.
Polymeric materials, developed for next-generation vaccine administration, generally act as delivery vehicles, allowing the controlled release of antigen and adjuvant over weeks to years, depending on the type of material. The antigen release rate can be controlled by tuning the material properties. The presence of degradable bonds in the polymer’s backbone and its hydrophilicity, molecular weight and crystallinity influence the degradation rate of both synthetic and natural polymers. For example, to enable instantaneous burst release, antigens can be encapsulated in rapidly dissolving water-soluble polymers, such as polyvinylpyrrolidone (PVP), which can be processed into microneedles for intradermal vaccine delivery117. A slow and sustained release can be achieved by choosing slowly degrading polymers, such as poly(lactic-co-glycolic acid) (PLGA)118. Increasing the window of antigen delivery can further improve the efficacy of vaccines119,120. By combining burst and slow-release strategies, single-dose regimens can be designed that mimic the multi-dose prime–boost regimen of conventional vaccines121; for example, a vaccine could be encapsulated in both a burst-release polymer and a slow-release polymer, which can then be simultaneously administered.
Delivery of adjuvants is key for vaccines. Some subunit vaccines, such as VLPs or recombinant VLPs and VNPs, which deliver subunit antigens, may not require additional adjuvants because the VLP or VNP itself acts as the adjuvant through recognition and activation of pattern recognition receptors122. However, for moderately or poorly immunogenic antigens, adjuvants need to be added to the formulation. The most frequently used adjuvants are aluminium-based salts (alum)113, which serve both as a delivery vehicle by adsorbing antigen to the surface and as an immunostimulator by inducing pro-inflammatory signalling cascades123. Similarly, polymers such as alginate, hyaluronic acid and PLGA can act as both delivery vehicle and adjuvant owing to their natural inflammatory properties124. Further activation of innate immunity can be achieved through co-delivery of Toll-like receptor (TLR) ligands, such as CpG-oligodeoxynucleotide or monophosphoryl lipid A. Here, materials design is crucial, because a growing body of data suggests that co-delivery of adjuvant and antigen to the same antigen-presenting cell is important to enhance vaccine efficacy and to increase safety by lowering the likelihood of an uncontrolled and unspecific systemic immune response125.
Vaccine delivery devices and production
As an alternative to subcutaneous injection with hypodermic needles, other routes of administration have been explored, using different device designs and fabrication, which have a considerable effect on immunostimulation. Here, we discuss two delivery constructs for vaccine self-administration: nano- and microparticle formulations, and microneedle patches. Using these devices, vaccines can be self-administered by intradermal, intranasal or oral administration (Fig. 4).
a | Number of research publications for microneedle, microparticle and nanoparticle vaccine delivery technologies. The terms ‘microneedle’, ‘microparticle’ and ‘nanoparticle’ were searched on PubMed, and the total number of publications for each search term were graphed with respect to publication date. b | Percentage distribution of biocompatible synthetic and natural polymers frequently used in research publications for vaccine delivery. The number of research publications on PubMed mentioning the respective polymers were counted for each polymer listed in the figure and totalled. The respective percentage of each polymer was then calculated from the total and graphed. c | Number of patent applications for microneedle, microparticle and nanoparticle vaccine delivery technologies. The terms ‘microneedle’, ‘microparticle’ and ‘nanoparticle’ were searched on www.uspto.gov, and the total number of publications for each search term was graphed with respect to publication date. d | Routes of administration for vaccine delivery proposed by patent applications. The number of patents mentioning the respective route of administration as well as the term “vaccine” were counted on www.uspto.gov and totalled. The respective percentage of each route of administration was then calculated from the total and graphed.
Nano- and microparticles
Vaccines can be encapsulated in polymeric nano- and microparticles. For example, polyester-based microparticles, such as PLGA microparticles, can be fabricated starting with an initial water-in-oil dispersion of polymer and antigen, followed by an additional emulsion and solvent removal step, polymer phase separation or spray drying126. By contrast, polyanhydrides require completely anhydrous conditions (solid–oil–oil dispersions) to prevent premature polymer hydrolysis127. Antigens can also be encapsulated in ionic polymers, such as polyphosphazenes and polysaccharides, by crosslinking of the polymers through the addition of oppositely charged multivalent ions in aqueous and ambient conditions. However, these fabrication techniques have several drawbacks, which can limit their use for certain biologics. For example, water-in-oil dispersion has low encapsulation efficiencies, which may limit its use for expensive and difficult-to-produce biologics128. Importantly, the fabrication process can affect antigen stability129; for example, antigens can degrade or unfold owing to interactions at the aqueous–organic interface or to high shear forces during emulsification130.
Nano- and microparticles are often used in self-administered intranasal vaccines. Here, antigens are encapsulated within nano- or microparticles, usually made of poly(lactic acid) or PLGA, and administered as a spray or aerosol, ideally targeting immune cells that reside in the nasal-associated lymphoid tissue of the upper respiratory tract131. However, following intranasal administration, the mucociliary clearance mechanism may clear the deposited vaccine particles, with a half-life of around 20 minutes132. To prevent rapid clearance, the vaccines can be formulated with mucoadhesive polymers, such as chitosan, which can translocate across the nasal mucosal barrier and prolong the residence time to hours or days to improve the immune response133.
Microneedles
Microneedles are microscale projections, usually 50 to 900 μm in height, which can pierce the upper layers of the skin and facilitate transdermal delivery of vaccines134. Microneedles do not puncture deep enough to activate the nociceptors within the skin and are therefore considered painless. Microneedles can achieve greater immunological responses, compared with traditional routes of administration, such as intramuscular or subcutaneous injection, owing to the abundant immune cell population in the upper layers of the skin, including the Langerhans cells in the epidermis and the dendritic cells in the dermis135,136. They can also be fabricated to deliver a range of biological vaccines, including inactivated and live attenuated viruses, VLPs and VNPs, protein subunits and DNA137. Importantly, encapsulation in microneeedle patches increases the thermostability of these biologics, which is particularly important for vaccination in regions without proper cold chain infrastructure. Therefore, microneedles are perhaps the most promising device for widespread vaccine distribution and self-administration.
Coated and dissolving microneedles have also been developed. Coated microneedles are designed by coating the surface of microneedles with antigen by dry-coating138 or layer-by-layer electrostatic deposition139. Upon skin puncture, the coated antigen is immediately released to elicit an immunological response. For dry-coating, a stainless steel or silicon microneedle is dip-coated or dry-sprayed with a mixed solution of antigen and carboxymethylcellulose140, polysaccharides141 or synthetic polymers142. A disadvantage of this method is its limited loading capacity134. However, loading can be increased by layer-by-layer electrostatic deposition; here, layers of oppositely charged polymers and proteins or nucleic acids are deposited in an alternating fashion139.
For the fabrication of dissolving microneedles, antigen is encapsulated within the microneedle tips, either in the bulk material or in microparticles. Upon insertion into the upper layers of the skin, the microneedles dissolve at rates dependent on the materials used143. Each material has advantages and disadvantages (Table 3); for example, PVP117 rapidly dissolves upon contact with the interstitial fluid, which decreases the application time of the microneedles, but requires high doses owing to fast clearance rates. Alternatively, chitosan biodegrades slowly, thereby acting as a sustained-release depot. Chitosan microneedles have been shown to induce a more potent and long-lasting immune response compared with conventional intramuscular injection or injection with alum121,144. Dissolving microneedles are usually manufactured by micromoulding145. Here, a mould is first filled with a polymer solution containing the antigen and then placed under vacuum or centrifuged to draw the solution into microcavities. The device is then allowed to dry and harden to form robust microneedle arrays. Water-soluble polymers, such as carboxymethyl cellulose and PVA, manufactured into microneedles under mild conditions, can protect encapsulated antigens from degradation146,147. Alternatively, dissolving microneedles can be fabricated by ultraviolet photo-polymerization of PVP within the mould117 or by high-temperature moulding148; however, these processes can cause antigen degradation, because they require harsher conditions than solvent-based moulding.
Microneedles with plant-produced antigens have already been shown to be effective. For example, a recombinant influenza VLP vaccine (along with a glucopyranosyl lipid adjuvant) administered by the NanoPass MicroJet microneedle, produced equal levels of haemagglutinin inhibition titres and seroprotection in humans, compared with intramuscular injection149. In ferrets, the microneedle formulation led to 100% survival against a H5N1 challenge. Microneedle delivery also enables in situ vaccination; for example, microneedle-delivered CPMV showed greater potency in a mouse model of melanoma compared with intratumoral injection of CPMV, resulting in slower tumour growth and improved overall survival150.
Outlook
The use of biologics in pharmaceutical applications will continue to grow as expression and purification technologies develop and mature. Plant molecular farming, in particular, is poised to make an impact on an array of applications, and new methodologies, such as the cookie technique, will continue to drive forward its use. Plant molecular farming is currently applied mainly in niche applications; however, recent developments and milestones, such as the production of millions of influenza vaccine doses in record time, has put a spotlight on this manufacturing platform45. Following such successes, it is likely that molecular farming will gain in market share. We have just witnessed the worldwide efforts by laboratories and industry in pivoting their particular platforms and tools towards making vaccines, therapeutics or reagents for the ongoing COVID-19 pandemic or the Zika and Ebola epidemics11,151. Plant molecular farming requires less sophisticated infrastructure than does conventional vaccine production, and thus could be locally deployed in each region, therefore addressing vaccine rollout challenges. Moreover, plant molecular farming could enable the production of vaccines, therapeutics or reagents in space, either on a space station, or during months-long missions to Mars or other extraterrestrial destinations152. The first grants supporting this technology have already been issued, for example, the NASA-funded use of transgenic lettuce and potato leaves to produce growth factors and hormones for astronauts to combat osteoporosis and other spaceflight-related health issues152.
However, expression levels in plant molecular farming must improve to levels similar to those achieved in mammalian or bacterial systems. Expression in bacterial systems was first achieved in the 1970s, ten years before expression in plants153. This gap means that plant molecular farming lags behind other expression systems in terms of expression efficiency, which will be addressed by more efficient plant systems that can produce proteins at larger yields. In addition, downstream processing and purification of biologics need to improve, which remains challenging owing to the complexity of plant cells and tissues (plants produce 30% more solid debris than other expression systems and contain many contaminants, such as phenolics, which interfere with purification). The FDA Critical Path Report states that downstream processes were one of the key reasons that plant-made proteins routinely failed to transition into the clinic or industry154. However, new methods, such as using acqueous two-phase partitioning systems, enzymatic hydrolysis, ultrasound, microwaves, pulsed electric fields, high-voltage electric discharge, ohmic heating, vacuum, subcritical and supercritical fluids, and hydrotropic and deep eutectic solvents are being developed to improve downstream processing of plant material155,156.
Manufacturing of biologics by plant molecular farming is currently performed mainly at small scales by academic research laboratories and mid-sized pharmaceutical companies, at a lower cost than traditional fermentation-based expression systems (up to ten times and a hundred times lower than the production costs in E. coli and CHO cells, respectively)157. Once plant molecular farming reaches agricultural crop scales, the margins are expected to widen even further. The lower costs will help to offset the expensive costs of everyday laboratory research. Thus, molecular farming has the potential to reduce the price of recombinant therapeutics to levels similar to those of everyday over-the-counter medications. Molecular farming could also play a key part in producing biosimilars (biologics that are not identical to the original product, but are equally efficacious) to fabricate cheap pharmaceuticals or biological test reagents for all populations. In addition, decreasing the price of medications would also greatly reduce financial strains on the health-care system. For example, the monoclonal antibodies used in the alleviation of rheumatoid arthritis are currently unaffordable outside health-care systems in developed regions, although the majority of sufferers remain in underdeveloped regions.
Future vaccines should be engineered to be free of cold chain restrictions, for example, by producing orally dosed vaccines and therapeutics in plants. Although the 1990s dream of simply ‘eating your vaccines’ is unattainable (mainly owing to low yield of active ingredient and dose control), transgenic plants consumed by humans, such as potatoes, tomatoes and corn, can be used to not only deliver partially purified and quantified antigen, but also to protect the antigen within a heat-stable environment158. The rigid plant cell wall can protect antigens from degradation within the harsh environment of the gastrointestinal system, while allowing antigen delivery159. The use of orally dosed vaccines can help reduce cold chain requirements and obviate the need for antigen purification and downstream processing62. The amount of land required to vaccinate entire countries by orally dosed vaccines would also be minimal (for example, all of China could be vaccinated with only 40 hectares of land)62.
Cold chain restrictions could also be mitigated by using new delivery agents, such as plant-made VNPs, that are intrinsically thermostable over a range of temperatures and pH levels, allowing their use in microneedle patches (which require high-temperature processing methods110), and in oral vaccines (avoiding denaturation by the acidic gastrointestinal environment160). Finally, new formulation chemistries and next-generation materials are anticipated to enhance formulation stability and improve delivery techniques. For example, the biomineralization of viral vaccines generates a mineral exterior, which improves the thermostability of the viral antigen161. Furthermore, new combinations of excipients can further extend the half-life of vaccines162. Finally, the formulation of biologics in microneedle patches and/or slow-release formulations has tremendous potential for the self-administration of medication.
Thus, platform technologies to produce biologics from living systems continue to develop as new technologies become available, and next-generation nanotechnology and materials science have opened up opportunities in formulation chemistry and the administration of pharmaceuticals.
References
Gurevich, E. V. & Gurevich, V. V. Beyond traditional pharmacology: new tools and approaches. Br. J. Pharmacol. 172, 3229–3241 (2015).
Tian, J. et al. Increased MSX level improves biological productivity and production stability in multiple recombinant GS CHO cell lines. Eng. Life Sci. 20, 112–125 (2020).
Chan, J. C. & Chan, A. T. Biologics and biosimilars: what, why and how? ESMO Open 2, e000180 (2017).
Orenstein, W. A. & Ahmed, R. Simply put: vaccination saves lives. Proc. Natl Acad. Sci. USA 114, 4031–4033 (2017).
Greenwood, B. The contribution of vaccination to global health: past, present and future. Phil. Trans. R. Soc. Lond. B 369, 20130433 (2014).
Ozawa, S. et al. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff. 35, 199–207 (2016).
Barta, A. et al. The expression of a nopaline synthase — human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant. Mol. Biol. 6, 347–357 (1986).
Ward, B. J. et al. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥65 years): two multicentre, randomised phase 3 trials. Lancet 396, 1491–1503 (2020). This paper describes an influenza vaccine produced in plants, which is one of the first adopted plant-derived pharmaceuticals.
[No authors listed.] In brief: Taliglucerase (Elelyso) for Gaucher disease. Med. Lett. Drugs Ther. 54, 56 (2012). This paper describes Elelyso, the first plant-derived human pharmaceutical on the market.
Rybicki, E. P. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. WIREs Nanomed. Nanobiol. 12, e1587 (2020).
Chung, Y. H., Beiss, V., Fiering, S. N. & Steinmetz, N. F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 14, 12522–12537 (2020).
Wang, J., Peng, Y., Xu, H., Cui, Z. & Williams, R. O. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 21, 225 (2020).
Plotkin, S. History of vaccination. Proc. Natl Acad. Sci. USA 111, 12283–12287 (2014).
Hoenig, L. J., Jackson, A. C. & Dickinson, G. M. The early use of Pasteur’s rabies vaccine in the United States. Vaccine 36, 4578–4581 (2018).
Enders, J. F., Weller, T. H. & Robbins, F. C. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science 109, 85–87 (1949).
Valenzuela, P., Medina, A., Rutter, W. J., Ammerer, G. & Hall, B. D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 298, 347–350 (1982).
Kaur, S. P. & Gupta, V. COVID-19 vaccine: a comprehensive status report. Virus Res. 288, 198114 (2020).
Delany, I., Rappuoli, R. & De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 6, 708–720 (2014).
Petrosky, E. et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR 64, 300–304 (2015).
Jang, Y. H. & Seong, B. L. The quest for a truly universal influenza vaccine. Front. Cell Infect. Microbiol. 9, 344 (2019).
Kines, R. C. et al. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLoS ONE 10, e0120797 (2015).
Shao, S., Ortega-Rivera, O. A., Ray, S. K., Pokorski, J. & Steinmetz, N. F. A scalable manufacturing approach to single dose vaccination against HPV. Vaccines 9, 66 (2021).
Rosenthal, K. S., Carambula, R. & Zimmerman, D. H. Why don’t we have a vaccine against autoimmune diseases? — a review. J. Clin. Cell Immunol. 10, 574 (2019).
Li, Q. & Ding, Z.-Y. The ways of isolating neoantigen-specific T cells. Front. Oncol. 10, 1347 (2020).
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2020).
Baeshen, N. A. et al. Cell factories for insulin production. Microb. Cell Fact. 13, 141 (2014).
Hammond, G. W. et al. Comparison of immunogenicity of two yeast-derived recombinant hepatitis B vaccines. Vaccine 9, 97–100 (1991).
Owczarek, B., Gerszberg, A. & Hnatuszko-Konka, K. A brief reminder of systems of production and chromatography-based recovery of recombinant protein biopharmaceuticals. Biomed. Res. Int. 2019, 4216060 (2019).
Overton, T. W. Recombinant protein production in bacterial hosts. Drug Discov. Today 19, 590–601 (2014).
Ferrer-Miralles, N., Domingo-Espín, J., Corchero, J. L., Vázquez, E. & Villaverde, A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact. 8, 17 (2009).
Makrides, S. C. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol. Mol. Biol. Rev. 60, 512–538 (1996).
Tripathi, N. K. & Shrivastava, A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front. Bioeng. Biotechnol. 7, 420 (2019).
Kim, J. Y., Kim, Y.-G. & Lee, G. M. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl. Microbiol. Biotechnol. 93, 917–930 (2012).
Dumont, J., Euwart, D., Mei, B., Estes, S. & Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit. Rev. Biotechnol. 36, 1110–1122 (2016).
Ghaderi, D., Zhang, M., Hurtado-Ziola, N. & Varki, A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol. Genet. Eng. Rev. 28, 147–175 (2012).
Jefferis, R. Posttranslational modifications and the immunogenicity of biotherapeutics. J. Immunol. Res. 2016, 5358272 (2016).
Ikonomou, L., Schneider, Y.-J. & Agathos, S. N. Insect cell culture for industrial production of recombinant proteins. Appl. Microbiol. Biotechnol. 62, 1–20 (2003).
Felberbaum, R. S. The baculovirus expression vector system: a commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 10, 702–714 (2015).
Rajaram, S., Boikos, C., Gelone, D. K. & Gandhi, A. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther. Adv. Vaccines Immunother. https://doi.org/10.1177/2515135520908121 (2020).
Izurieta, H. S. et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J. Infect. Dis. 220, 1255–1264 (2019).
Gregersen, J.-P. A risk-assessment model to rate the occurrence and relevance of adventitious agents in the production of influenza vaccines. Vaccine 26, 3297–3304 (2008).
Rybicki, E. P. Plant-made vaccines for humans and animals. Plant. Biotechnol. J. 8, 620–637 (2010). This paper gives an overview of molecular farming innovations for vaccines in both humans and animals.
Pujol, M. et al. An integral approach towards a practical application for a plant-made monoclonal antibody in vaccine purification. Vaccine 23, 1833–1837 (2005).
Liew, P. S. & Hair-Bejo, M. Farming of plant-based veterinary vaccines and their applications for disease prevention in animals. Adv. Virol. 2015, 936940 (2015).
Lico, C. et al. Plant molecular farming as a strategy against COVID-19 — the Italian perspective. Front. Plant. Sci. 11, 609910 (2020).
Mahmood, N., Nasir, S. B. & Hefferon, K. Plant-based drugs and vaccines for COVID-19. Vaccines 9, 15 (2020). This paper highlights some of the drugs and vaccines produced through molecular farming in the fight against COVID-19, with specific mention of Medicago.
Ward, B. J. et al. Phase 1 trial of a candidate recombinant virus-like particle vaccine for Covid-19 disease produced in plants. Preprint at medRxiv https://doi.org/10.1101/2020.11.04.20226282 (2020).
Kyriakidis, N. C., López-Cortés, A., González, E. V., Grimaldos, A. B. & Prado, E. O. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines 6, 1–17 (2021).
Doron, L., Segal, N. & Shapira, M. Transgene expression in microalgae — from tools to applications. Front. Plant. Sci. 7, 505 (2016).
Dyo, Y. M. & Purton, S. The algal chloroplast as a synthetic biology platform for production of therapeutic proteins. Microbiology 164, 113–121 (2018).
Taunt, H. N., Stoffels, L. & Purton, S. Green biologics: the algal chloroplast as a platform for making biopharmaceuticals. Bioengineered 9, 48–54 (2018).
Gasdaska, J., Spencer, D. & Dickey, L. Advantages of therapeutic protein production in the aquatic plant Lemna. BioProcess. J. 2, 49–56 (2003).
Reski, R., Parsons, J. & Decker, E. L. Moss-made pharmaceuticals: from bench to bedside. Plant. Biotechnol. J. 13, 1191–1198 (2015).
Park, K. Y. & Wi, S. J. Potential of plants to produce recombinant protein products. J. Plant. Biol. 59, 559–568 (2016). This paper provides a broad overview of the current industry of plant-produced biologics, highlighting the benefits compared with other expression systems as well as the challenges associated with plant molecular farming.
Rybicki, E. P. Plant-produced vaccines: promise and reality. Drug Discov. Today 14, 16–24 (2009).
Tschofen, M., Knopp, D., Hood, E. & Stöger, E. Plant molecular farming: much more than medicines. Annu. Rev. Anal. Chem. 9, 271–294 (2016).
Moon, K.-B. et al. Development of systems for the production of plant-derived biopharmaceuticals. Plants 9, 30 (2019).
Shaaltiel, Y. et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant. Biotechnol. J. 5, 579–590 (2007).
Landry, N. et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 5, e15559 (2010).
Margolin, E. et al. Co-expression of human calreticulin significantly improves the production of HIV gp140 and other viral glycoproteins in plants. Plant. Biotechnol. J. 18, 2109–2117 (2020).
Merlin, M., Gecchele, E., Capaldi, S., Pezzotti, M. & Avesani, L. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. BioMed. Res. Int. 2014, 136419 (2014).
Gunasekaran, B. & Gothandam, K. M. A review on edible vaccines and their prospects. Braz. J. Med. Biol. Res. 53, e8749 (2020).
Naupu, P. N., van Zyl, A. R., Rybicki, E. P. & Hitzeroth, I. I. Immunogenicity of plant-produced human papillomavirus (HPV) virus-like particles (VLPs). Vaccines 8, 740 (2020).
Lai, H. et al. A plant-produced vaccine protects mice against lethal West Nile virus infection without enhancing Zika or dengue virus infectivity. Vaccine 36, 1846–1852 (2018).
Ponndorf, D. et al. Plant-made dengue virus-like particles produced by co-expression of structural and non-structural proteins induce a humoral immune response in mice. Plant. Biotechnol. J. 19, 745–756 (2021).
Lamprecht, R. L. et al. Production of human papillomavirus pseudovirions in plants and their use in pseudovirion-based neutralisation assays in mammalian cells. Sci. Rep. 6, 20431 (2016).
Zhou, Y., Maharaj, P. D., Mallajosyula, J. K., McCormick, A. A. & Kearney, C. M. In planta production of flock house virus transencapsidated RNA and its potential use as a vaccine. Mol. Biotechnol. 57, 325–336 (2015).
Xu, J. & Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: present status and prospect. Pharm. Bioprocess. 2, 499–518 (2014).
Rademacher, T. et al. Plant cell packs: a scalable platform for recombinant protein production and metabolic engineering. Plant. Biotechnol. J. 17, 1560–1566 (2019).
Gengenbach, B. B., Opdensteinen, P. & Buyel, J. F. Robot cookies — plant cell packs as an automated high-throughput screening platform based on transient expression. Front. Bioeng. Biotechnol. 8, 393 (2020). This paper describes the cookie technique for infiltrating plant cells with Agrobacterium suspensions, resulting in automated, high-throughput production of biologics.
Pishko, A. & Nasta, S. D. The role of novel immunotherapies in non-Hodgkin lymphoma. Transl. Cancer Res. 6, 93–103 (2017).
McCormick, A. A. Tobacco derived cancer vaccines for non-Hodgkin’s lymphoma: perspectives and progress. Hum. Vaccin. 7, 305–312 (2011).
Tusé, D. et al. Clinical safety and immunogenicity of tumor-targeted, plant-made Id-KLH conjugate vaccines for follicular lymphoma. Biomed. Res. Int. 2015, 648143 (2015).
Rybicki, E. P. Plant-made vaccines and reagents for the One Health initiative. Hum. Vaccin. Immunother. 13, 2912–2917 (2017).
Atkinson, R., Burt, F., Rybicki, E. P. & Meyers, A. E. Plant-produced Crimean–Congo haemorrhagic fever virus nucleoprotein for use in indirect ELISA. J. Virol. Methods 236, 170–177 (2016).
Mbewana, S. et al. Expression of Rift Valley fever virus N-protein in Nicotiana benthamiana for use as a diagnostic antigen. BMC Biotechnol. 18, 77 (2018).
Margolin, E. et al. Calreticulin co-expression supports high level production of a recombinant SARS-CoV-2 spike mimetic in Nicotiana benthamiana. Preprint at bioRxiv https://doi.org/10.1101/2020.06.14.150458 (2020).
Steele, J. F. C. et al. Synthetic plant virology for nanobiotechnology and nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9, e1447 (2017).
Czapar, A. E. & Steinmetz, N. F. Plant viruses and bacteriophages for drug delivery in medicine and biotechnology. Curr. Opin. Chem. Biol. 38, 108–116 (2017).
Shoeb, E. & Hefferon, K. Future of cancer immunotherapy using plant virus-based nanoparticles. Future Sci. OA 5, FSO401 (2019).
Chan, S. K. et al. Biomimetic virus-like particles as severe acute respiratory syndrome coronavirus 2 diagnostic tools. ACS Nano 15, 1259–1272 (2020).
Chung, Y. H., Cai, H. & Steinmetz, N. F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 156, 214–235 (2020). This paper provides an in-depth overview of viral nanoparticles (with a focus on plant viral nanoparticles) used in drug delivery, imaging, immunotherapy and theranostics.
Wi, S., Hwang, I. S. & Jo, B. H. Engineering a plant viral coat protein for in vitro hybrid self-assembly of CO2-capturing catalytic nanofilaments. Biomacromolecules 21, 3847–3856 (2020).
Hu, H. et al. Physalis mottle virus-like nanoparticles for targeted cancer imaging. ACS Appl. Mater. Interfaces 11, 18213–18223 (2019).
Chariou, P. L. & Steinmetz, N. F. Delivery of pesticides to plant parasitic nematodes using tobacco mild green mosaic virus as a nanocarrier. ACS Nano 11, 4719–4730 (2017).
Barone, P. W. et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat. Biotechnol. 38, 563–572 (2020).
Lebel, M.-È., Chartrand, K., Leclerc, D. & Lamarre, A. Plant viruses as nanoparticle-based vaccines and adjuvants. Vaccines 3, 620–637 (2015).
Kerstetter-Fogle, A. et al. Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers 11, 515 (2019).
Shukla, S., Wang, C., Beiss, V. & Steinmetz, N. F. Antibody response against cowpea mosaic viral nanoparticles improves in situ vaccine efficacy in ovarian cancer. ACS Nano 14, 2994–3003 (2020).
Murray, A. A., Wang, C., Fiering, S. & Steinmetz, N. F. In situ vaccination with cowpea vs tobacco mosaic virus against melanoma. Mol. Pharm. 15, 3700–3716 (2018).
Cai, H., Wang, C., Shukla, S. & Steinmetz, N. F. Cowpea mosaic virus immunotherapy combined with cyclophosphamide reduces breast cancer tumor burden and inhibits lung metastasis. Adv. Sci. 6, 1802281 (2019).
Lizotte, P. H. et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 11, 295–303 (2016).
Hoopes, P. J. et al. Treatment of canine oral melanoma with nanotechnology-based immunotherapy and radiation. Mol. Pharmaceutics. 15, 3717–3722 (2018).
Evtushenko, E. A., Ryabchevskaya, E. M., Nikitin, N. A., Atabekov, J. G. & Karpova, O. V. Plant virus particles with various shapes as potential adjuvants. Sci. Rep. 10, 10365 (2020).
Shukla, S. et al. Plant viruses and bacteriophage-based reagents for diagnosis and therapy. Annu. Rev. Virol. 7, 559–587 (2020).
Ortega-Rivera, O. A. et al. Trivalent subunit vaccine candidates for COVID-19 and their delivery devices. J. Am. Chem. Soc. 143, 14748–14765 (2021).
Shen, A. K., Fields, R. & McQuestion, M. The future of routine immunization in the developing world: challenges and opportunities. Glob. Health Sci. Pract. 2, 381–394 (2014).
Kristensen, D. D., Lorenson, T., Bartholomew, K. & Villadiego, S. Can thermostable vaccines help address cold-chain challenges? Results from stakeholder interviews in six low- and middle-income countries. Vaccine 34, 899–904 (2016).
Zaffran, M. et al. The imperative for stronger vaccine supply and logistics systems. Vaccine 31, B73–B80 (2013).
Eriksson, P., Gessner, B. D., Jaillard, P., Morgan, C. & Le Gargasson, J. B. Vaccine vial monitor availability and use in low- and middle-income countries: a systematic review. Vaccine 35, 2155–2161 (2017).
Halm, A. et al. Using oral polio vaccine beyond the cold chain: a feasibility study conducted during the national immunization campaign in Mali. Vaccine 28, 3467–3472 (2010).
Zipursky, S. et al. Assessing the potency of oral polio vaccine kept outside of the cold chain during a national immunization campaign in Chad. Vaccine 29, 5652–5656 (2011).
Chen, D. & Kristensen, D. Opportunities and challenges of developing thermostable vaccines. Expert. Rev. Vaccines 8, 547–557 (2009).
Ngabo, F. et al. A cost comparison of introducing and delivering pneumococcal, rotavirus and human papillomavirus vaccines in Rwanda. Vaccine 33, 7357–7363 (2015).
Bahamondes, L. et al. Uniject as a delivery system for the once-a-month injectable contraceptive Cyclofem in Brazil. Contraception 53, 115–119 (1996).
Sutanto, A., Suarnawa, I. M., Nelson, C. M., Stewart, T. & Soewarso, T. I. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull. WHO 77, 119–126 (1999).
Dyer, O. Covid-19: many poor countries will see almost no vaccine next year, aid groups warn. BMJ 371, m4809 (2020).
Ma, J. K.-C. et al. Realising the value of plant molecular pharming to benefit the poor in developing countries and emerging economies. Plant. Biotechnol. J. 11, 1029–1033 (2013). This paper highlights how plant molecular farming could especially benefit and improve the health of citizens in low- to middle-income areas through a four-point plan.
Madi, M., Mioulet, V., King, D. P., Lomonossoff, G. P. & Montague, N. P. Development of a non-infectious encapsidated positive control RNA for molecular assays to detect foot-and-mouth disease virus. J. Virol. Methods 220, 27–34 (2015).
Wang, Q., Lin, T., Tang, L., Johnson, J. E. & Finn, M. G. Icosahedral virus particles as addressable nanoscale building blocks. Angew. Chem. Int. Ed. 41, 459–462 (2002).
Kossover-Smith, R. A. et al. One needle, one syringe, only one time? A survey of physician and nurse knowledge, attitudes, and practices around injection safety. Am. J. Infect. Control. 45, 1018–1023 (2017).
Nir, Y., Paz, A., Sabo, E. & Potasman, I. Fear of injections in young adults: prevalence and associations. Am. J. Trop. Med. Hyg. 68, 341–344 (2003).
Kumru, O. S. et al. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals 42, 237–259 (2014).
Hageman, M. J. The role of moisture in protein stability. Drug Dev. Ind. Pharm. 14, 2047–2070 (1988).
Ulery, B. D., Nair, L. S. & Laurencin, C. T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B 49, 832–864 (2011). This paper provides an overview of different polymers and their respective contributions to the biomedical fields of drug delivery and tissue engineering.
Lee, P. W. et al. Biodegradable viral nanoparticle/polymer implants prepared via melt-processing. ACS Nano 11, 8777–8789 (2017).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 16, 915–920 (2010).
Makadia, H. K. & Siegel, S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011).
Tam, H. H. et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl Acad. Sci. 113, E6639–E6648 (2016).
Kapadia, C. H. et al. Extending antigen release from particulate vaccines results in enhanced antitumor immune response. J. Control. Release 269, 393–404 (2018).
Walters, A. A., Krastev, C., Hill, A. V. S. & Milicic, A. Next generation vaccines: single-dose encapsulated vaccines for improved global immunisation coverage and efficacy. J. Pharm. Pharmacol. 67, 400–408 (2015).
Mohsen, M. O., Gomes, A. C., Vogel, M. & Bachmann, M. F. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 6, 37 (2018).
Reed, S. G., Bertholet, S., Coler, R. N. & Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 30, 23–32 (2009).
Shields, C. W., Wang, L. L., Evans, M. A. & Mitragotri, S. Materials for immunotherapy. Adv. Mater. 32, 1901633 (2020).
Jones, K. S. Biomaterials as vaccine adjuvants. Biotechnol. Progress. 24, 807–814 (2008).
Jain, R., Shah, N. H., Malick, A. W. & Rhodes, C. T. Controlled drug delivery by biodegradable poly(ester) devices: different preparative approaches. Drug Dev. Ind. Pharm. 24, 703–727 (1998).
Kumar, N., Langer, R. S. & Domb, A. J. Polyanhydrides: an overview. Adv. Drug Deliv. Rev. 54, 889–910 (2002).
Chen, J. L., Chiang, C. H. & Yeh, M. K. The mechanism of PLA microparticle formation by water-in-oil-in-water solvent evaporation method. J. Microencapsul. 19, 333–346 (2002).
van de Weert, M., Hennink, W. E. & Jiskoot, W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 17, 1159–1167 (2000).
Sah, H. Protein instability toward organic solvent/water emulsification: implications for protein microencapsulation into microspheres. PDA J. Pharm. Sci. Technol. 53, 3–10 (1999).
Illum, L., Jabbal-Gill, I., Hinchcliffe, M., Fisher, A. N. & Davis, S. S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 51, 81–96 (2001).
Soane, R. J. et al. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 178, 55–65 (1999).
Slütter, B., Hagenaars, N. & Jiskoot, W. Rational design of nasal vaccines. J. Drug Target. 16, 1–17 (2008).
Yang, J., Liu, X., Fu, Y. & Song, Y. Recent advances of microneedles for biomedical applications: drug delivery and beyond. Acta Pharm. Sin. B 9, 469–483 (2019). This Review highlights recent developments in microneedle drug delivery applications in the fields of therapeutics, vaccines, diagnosis and cosmetics, and compares and contrasts the different types of microneedles.
Nestle, F. O., Meglio, P. D., Qin, J.-Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Menon, I. et al. Microneedles: a new generation vaccine delivery system. Micromachines 12, 435 (2021).
Li, J., Zeng, M., Shan, H. & Tong, C. Microneedle patches as drug and vaccine delivery platform. Curr. Med. Chem. 24, 2413–2422 (2017).
Kim, Y.-C., Quan, F.-S., Compans, R. W., Kang, S.-M. & Prausnitz, M. R. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release 142, 187–195 (2010).
DeMuth, P. C., Moon, J. J., Suh, H., Hammond, P. T. & Irvine, D. J. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano 6, 8041–8051 (2012).
Gill, H. S. & Prausnitz, M. R. Coated microneedles for transdermal delivery. J. Control. Release 117, 227–237 (2007).
Choi, H.-J. et al. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. J. Control. Release 166, 159–171 (2013).
Andrianov, A. K. & Mutwiri, G. Intradermal immunization using coated microneedles containing an immunoadjuvant. Vaccine 30, 4355–4360 (2012).
Donnelly, R. F., Raj Singh, T. R. & Woolfson, A. D. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 17, 187–207 (2010).
Chen, M.-C., Huang, S.-F., Lai, K.-Y. & Ling, M.-H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 34, 3077–3086 (2013).
Hong, X. et al. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Devel. Ther. 7, 945–952 (2013).
Bachy, V. et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc. Natl Acad. Sci. USA 110, 3041–3046 (2013).
Edens, C., Collins, M. L., Goodson, J. L., Rota, P. A. & Prausnitz, M. R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 33, 4712–4718 (2015).
Park, J.-H., Allen, M. G. & Prausnitz, M. R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 23, 1008–1019 (2006).
Carter, D. et al. The adjuvant GLA-AF enhances human intradermal vaccine responses. Sci. Adv. 4, eaas9930 (2018).
Boone, C. E. et al. Active microneedle administration of plant virus nanoparticles for cancer in situ vaccination improves immunotherapeutic efficacy. ACS Appl. Nano Mater. 3, 8037–8051 (2020).
Bamogo, P. K. A. et al. Virus-based pharmaceutical production in plants: an opportunity to reduce health problems in Africa. Virol. J. 16, 167 (2019).
McNulty, M. J. et al. Molecular pharming to support human life on the Moon, Mars, and beyond. Crit. Rev. Biotechnol. 41, 849–864 (2020).
Davies, R., Hardern, I. & Overman, R. A history of recombinant protein technology in small molecule drug discovery. Eur. Pharm. Rev. https://www.europeanpharmaceuticalreview.com/article/27775/history-recombinant-protein-technology-small-molecule-drug-discovery/ (2014).
Lico, C., Chen, Q. & Santi, L. Viral vectors for production of recombinant proteins in plants. J. Cell Physiol. 216, 366–377 (2008).
Platis, D. & Labrou, N. E. Development of an aqueous two-phase partitioning system for fractionating therapeutic proteins from tobacco extract. J. Chromatogr. A. 1128, 114–124 (2006).
Gençdag˘, E., Görgüç, A. & Yılmaz, F. M. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Int. 37, 447–468 (2021).
Fischer, R., Drossard, J., Commandeur, U., Schillberg, S. & Emans, N. Towards molecular farming in the future: moving from diagnostic protein and antibody production in microbes to plants. Biotechnol. Appl. Biochem. 30, 101–108 (1999).
Daniell, H., Streatfield, S. J. & Wycoff, K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant. Sci. 6, 219–226 (2001).
Sahai, A., Shahzad, A. & Shahid, M. in Recent Trends in Biotechnology and Therapeutic Applications of Medicinal Plants (eds Shahid, M., Shahzad, A., Malik, A., Sahai, A.) 225–252 (Springer Netherlands, 2013).
Berardi, A., Evans, D. J., Bombelli, F. B. & Lomonossoff, G. P. Stability of plant virus-based nanocarriers in gastrointestinal fluids. Nanoscale 10, 1667–1679 (2018).
Wang, G. et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl Acad. Sci. USA 110, 7619–7624 (2013).
Wiggan, O. et al. Novel formulations enhance the thermal stability of live-attenuated flavivirus vaccines. Vaccine 29, 7456–7462 (2011).
Lievano, F. et al. Measles, mumps, and rubella virus vaccine (M-M-RTMII): a review of 32 years of clinical and postmarketing experience. Vaccine 30, 6918–6926 (2012).
Van Herck, K. & Van Damme, P. Prevention of hepatitis A by Havrix: a review. Expert Rev. Vaccines 4, 459–471 (2005).
Van Damme, P. et al. Safety, tolerability and immunogenicity of a recombinant hepatitis B vaccine manufactured by a modified process in healthy young adults. Hum. Vaccin 5, 92–97 (2009).
Cheng, L., Wang, Y. & Du, J. Human papillomavirus vaccines: an updated review. Vaccines 8, 391 (2020).
Pollet, J., Chen, W.-H. & Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 170, 71–82 (2021).
Bhuyan, P. K. et al. Durability of response to VGX-3100 treatment of HPV16/18 positive cervical HSIL. Hum. Vaccin. Immunother. 17, 1288–1293 (2020).
Wolf, J. et al. Development of pandemic vaccines: ERVEBO case study. Vaccines 9, 190 (2021).
Tregoning, J. S. First human efficacy study of a plant-derived influenza vaccine. Lancet 396, 1464–1465 (2020).
Li, T., Sun, J., Lu, Z. & Liu, Q. Transformation of HBsAg (hepatitis B surface antigen) gene into tomato mediated by Agrobacterium tumefaciens. Czech J. Genet. Plant. Breed. 47, 69–77 (2011).
Rosales-Mendoza, S. et al. Expression of Escherichia coli heat-labile enterotoxin b subunit (LTB) in carrot (Daucus carota L.). Plant. Cell Rep. 26, 969–976 (2007).
Wu, Y.-Z. et al. Oral immunization with rotavirus VP7 expressed in transgenic potatoes induced high titers of mucosal neutralizing IgA. Virology 313, 337–342 (2003).
Phoolcharoen, W. et al. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant. Biotechnol. J. 9, 807–816 (2011).
Li, Y. et al. High expression of foot-and-mouth disease virus structural protein VP1 in tobacco chloroplasts. Plant. Cell Rep. 25, 329–333 (2006).
Jones, R. M. et al. A Plant-Produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS ONE 8, e79538 (2013).
Lai, H. & Chen, Q. Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current good manufacture practice regulations. Plant. Cell Rep. 31, 573–584 (2012).
Kim, M.-Y., Yang, M.-S. & Kim, T.-G. Expression of dengue virus E glycoprotein domain III in non-nicotine transgenic tobacco plants. Biotechnol. Bioproc. E 14, 725–730 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04450004 (2020).
Ceballo, Y. et al. High accumulation in tobacco seeds of hemagglutinin antigen from avian (H5N1) influenza. Transgenic Res. 26, 775–789 (2017).
Olinger, G. G. et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc. Natl Acad. Sci. USA 109, 18030–18035 (2012).
Penney, C. A., Thomas, D. R., Deen, S. S. & Walmsley, A. M. Plant-made vaccines in support of the millennium development goals. Plant Cell Rep. 30, 789–798 (2011).
Atsmon, J. et al. Preclinical and first-in-human evaluation of PRX-105, a PEGylated, plant-derived, recombinant human acetylcholinesterase-R. Toxicol. Appl. Pharmacol. 287, 202–209 (2015).
Bethell, D. R. & Huang, J. Recombinant human lactoferrin treatment for global health issues: iron deficiency and acute diarrhea. Biometals 17, 337–342 (2004).
Shanmugaraj, B., Rattanapisit, K., Manopwisedjaroen, S., Thitithanyanont, A. & Phoolcharoen, W. Monoclonal antibodies B38 and H4 produced in Nicotiana benthamiana neutralize SARS-CoV-2 in vitro. Front. Plant Sci. 11, 589995 (2020).
Rattanapisit, K. et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 10, 17698 (2020).
Tekoah, Y. et al. Large-scale production of pharmaceutical proteins in plant cell culture — the protalix experience. Plant. Biotechnol. J. 13, 1199–1208 (2015).
Perea Arango, I. et al. Expression of the rabies virus nucleoprotein in plants at high-levels and evaluation of immune responses in mice. Plant. Cell Rep. 27, 677–685 (2008).
Woodruff, M. A. & Hutmacher, D. W. The return of a forgotten polymer — polycaprolactone in the 21st century. Prog. Polym. Sci. 35, 1217–1256 (2010).
da Silva, D. et al. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 340, 9–14 (2018).
Teasdale, I. & Brüggemann, O. Polyphosphazenes: multifunctional, biodegradable vehicles for drug and gene delivery. Polymers 5, 161–187 (2013).
Fakhari, A. & Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 9, 7081–7092 (2013).
Klotz, U. & Kroemer, H. Clinical pharmacokinetic considerations in the use of plasma expanders. Clin. Pharmacokinet. 12, 123–135 (1987).
Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
Chu, L. Y., Choi, S.-O. & Prausnitz, M. R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J. Pharm. Sci. 99, 4228–4238 (2010).
Low, L.-Y. et al. in New Visions in Plant Science (ed. Celik, O) Ch. 3 (InTechOpen, 2018).
Ochoa-Villarreal, M. et al. Plant cell culture strategies for the production of natural products. BMB Rep. 49, 149–158 (2016).
Torti, S. et al. Transient reprogramming of crop plants for agronomic performance. Nat. Plants 7, 159–171 (2021).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Acknowledgements
This work was funded in part by the following grants from the NIH (U01-CA218292, R01-CA224605, R01-CA253615, R01-CA202-814 and R01-HL137674 to N.F.S.; R21AI161306 to N.F.S.), the American Cancer Society (128319-RSG-15-144-01-CDD to N.F.S.), the USDA (NIFA-2020-67021-31255 to N.F.S.), the CDMRP (W81XWH2010742 to N.F.S.), the NSF (RAPID CBET-2032196 to N.F.S.; RAPID CMMI-2027668 to N.F.S. and J.K.P.) as well as partially supported by the NSF through the UC San Diego Materials Research Science and Engineering Center (UCSD MRSEC), grant DMR-2011924 (to N.F.S., J.K.P., Y.C., D.C.). E.P.R. acknowledges research support from Medicago Inc. (Canada), the South African Medical Research Council and National Research Foundation, and the Poliomyelitis Research Foundation. E.C.K. was supported by the NIH T32EB005970 institutional training grant through the Clinician-Scientist Radiology Residency Program. E.O. was supported by the NIH 2T32 CA153915-06A1 institutional training grant through the Cancer Research in Nanotechnology San Diego Fellowship.
Author information
Authors and Affiliations
Contributions
Y.C. wrote the first drafts of the manuscript, researched data for the article and prepared figures; D.C. wrote the sections focusing on vaccine administration technology and prepared figures; E.C.K. wrote the sections focusing on biologics and background on vaccines and prepared figures; E.O. wrote the sections focusing on the cold chain and vaccine distribution and prepared figures; S.S. wrote the sections focusing on molecular farming for plant viral nanoparticles and prepared figures. E.P.R. and N.F.S. wrote the section on plant molecular farming. N.F.S. and Y.C. prepared the final version of the manuscript. N.F.S. and J.K.P. conceived the manuscript and were in charge of overall direction and planning. All authors reviewed and edited the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.F.S. and J.K.P. are co-founders of and have a financial interest with Mosaic IE Inc. E.P.R. has a financial interest in Cape Bio Pharms Ltd in Cape Town. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Materials thanks Krishnendu Roy and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
The World Health Organization (WHO): https://www.who.int/immunization/call-to-action_ipac-iscl.pdf
Rights and permissions
About this article
Cite this article
Chung, Y.H., Church, D., Koellhoffer, E.C. et al. Integrating plant molecular farming and materials research for next-generation vaccines. Nat Rev Mater 7, 372–388 (2022). https://doi.org/10.1038/s41578-021-00399-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00399-5
This article is cited by
-
Risk assessment and bioburden evaluation of Agrobacterium tumefaciens-mediated transient protein expression in plants using the CaMV35S promoter
BMC Biotechnology (2023)
-
Engineering protein-based therapeutics through structural and chemical design
Nature Communications (2023)
-
The emerging role of nanotechnology in plant genetic engineering
Nature Reviews Bioengineering (2023)
-
Plant-based biopharmaceutical engineering
Nature Reviews Bioengineering (2023)
-
Extraction and purification of malaria vaccine candidate CLCT produced by transient expression in Nicotiana benthamiana plants
Discover Chemical Engineering (2023)