Abstract
Direct measurement of methane emissions is cost-prohibitive for greenhouse gas offset projects, necessitating the development of alternative accounting methods such as proxies. Salinity is a useful proxy for tidal marsh CH4 emissions when comparing across a wide range of salinity regimes but does not adequately explain variation in brackish and freshwater regimes, where variation in emissions is large. We sought to improve upon the salinity proxy in a marsh complex on Deal Island Peninsula, Maryland, USA by comparing emissions from four strata differing in hydrology and plant community composition. Mean CH4 chamber-collected emissions measured as mg CH4 m−2 h−1 ranked as S. alterniflora (1.2 ± 0.3) ≫ High-elevation J. roemerianus (0.4 ± 0.06) > Low-elevation J. roemerianus (0.3 ± 0.07) = S. patens (0.1 ± 0.01). Sulfate depletion generally reflected the same pattern with significantly greater depletion in the S. alterniflora stratum (61 ± 4%) than in the S. patens stratum (1 ± 9%) with the J. roemerianus strata falling in between. We attribute the high CH4 emissions in the S. alterniflora stratum to sulfate depletion likely driven by limited connectivity to tidal waters. Low CH4 emissions in the S. patens stratum are attributed to lower water levels, higher levels of ferric iron, and shallow rooting depth. Moderate CH4 emissions from the J. roemerianus strata were likely due to plant traits that favor CH4 oxidation over CH4 production. Hydrology and plant community composition have significant potential as proxies to estimate CH4 emissions at the site scale.
Similar content being viewed by others
Introduction
Methane is a potent greenhouse gas produced under the dominantly anaerobic conditions found in wetland soils. The global warming potential of methane (CH4) gas is 32–45 times greater than an equivalent amount of carbon dioxide (CO2) over a 100-year period (Neubauer and Megonigal 2015). While the majority of CH4 emissions come from anthropogenic sources, wetlands produce most of the naturally emitted CH4 (Wang et al. 1996; Solomon et al. 2007) and are the most important source of uncertainty in current global CH4 budgets (Saunois et al. 2020). Coastal wetland CH4 emissions were recently estimated at 5.3–6.2 Tg CH4 year−1, amounting to 60% of the global marine CH4 budget (Al-Haj and Fulweiler 2020), and < 7% of the global wetland CH4 budget (Saunois et al. 2020). Methane emissions are the largest source of uncertainty in the coastal wetland greenhouse gas budget (Holmquist et al. 2018). There is emerging interest in using tidal marsh restoration and conservation to mitigate greenhouse gases in the atmosphere and as a source of carbon credits (Crooks et al. 2011; Emmer et al. 2015a, b; Needelman et al. 2018; Emmer et al. 2020a,b), but the high carbon sequestration rates characteristic of tidal wetlands soils (Chmura et al. 2003; Nahlik and Fennesy 2016) can be partly or completely offset by CH4 emissions (Poffenbarger et al. 2011). The uncertainty introduced to greenhouse gas offset activities by CH4 emissions is especially large for coastal wetlands ecosystems with freshwater-to-brackish salinity < 18 ppt (Poffenbarger et al. 2011). The sources of this variability remain elusive as there has been relatively little research designed to partition variation. Direct monitoring of methane emissions is cost-prohibitive for most blue carbon crediting projects (Needelman et al. 2018), creating a need for a better understanding of the factors that regulate coastal wetland CH4 emissions to create alternative estimation methods such as proxies and models.
Methane is produced in wetlands by methanogenic archaea. The production of CH4 occurs when there is an excess of electron donors over electron acceptors, depleting the availability of alternative electron acceptors such as ferric iron (Fe(III)) and sulfate (SO42−) (Megonigal et al. 2004). Electron donors are produced from labile organic materials that undergo fermentation to low molecular weight carbon compounds and H2. Electron donors can be present in the soil (e.g. Fe(III)), supplied from external sources such as floodwater (e.g. SO42−), or provided by plants (e.g. molecular oxygen or oxidized compounds generated by radial oxygen loss). The availability of SO42− from seawater suppresses CH4 emissions from polyhaline (salinity > 18 ppt) marshes to consistently low rates (0.2 to 5.7 g CH4 m−2 year−1) (Poffenbarger et al. 2011), although high emission rates have been observed in polyhaline wetlands with high anthropogenic inputs (Purvaja and Ramesh 2001) and low SO42− replenishment due to restricted tidal exchange (Emery and Fulweiler 2017). Methane emissions from mesohaline brackish systems (5–18 ppt salinity) are greater and more variable (3.3 to 32.0 g CH4 m−2 year−1). Methanogenesis is also regulated by such physiochemical factors as pH (Walker et al. 1998; Garcia et al. 2000) and temperature (Megonigal and Schlesinger 2002; Whalen 2005).
Differences in methane emissions between dominant vegetation communities have been observed in tidal marsh systems such as Phragmites australis versus S. alterniflora (Yuan et al. 2015), P. australis versus mixed and native zones (Mueller et al. 2016) and S. patens versus Schoenoplectus americanus (Noyce and Megonigal 2021); however, such differences are not always observed (Emery and Fulweiler 2014). Plant species composition affects CH4 emissions through several mechanisms (Koebsch et al. 2013; Moor et al. 2017; Mueller et al. 2020). The availability of electron donors is largely determined by primary productivity which varies with species composition (Megonigal et al. 2004). Species composition also regulates electron acceptor availability through rhizosphere processes such as root oxygen loss (Calhoun and King, 1997; Jespersen et al. 1998; Colmer 2003) and rhizosphere regeneration of ferric iron (Neubauer et al, 2005; Sutton-Grier and Megonigal, 2011). Methane can be transported to the atmosphere via aerenchyma tissue, bypassing the emission barriers caused by slow CH4 diffusion rates through soils and soil-surface CH4 oxidation zones (Le Mer and Roger 2001; Ding et al. 2005; Sorrell et al. 2013; Villa et al. 2020). This is important because CH4 oxidation can consume 70% or more of the CH4 produced in tidal wetland soils (Megonigal and Schlesinger 2002).
In tidal marshes, water table position and periods of soil inundation are controlled by hydrologic factors such as tides, soil hydraulic conductivity, distance from open water, and soil surface elevation relative to sea surface elevation. Elevation zonation subdivides tidal marshes into low marsh areas that are frequently inundated by the tides, and high marsh areas that are infrequently influenced by the tides. Water table depth influences soil oxygen availability (Epp and Chanton 1993; Gilbert and Frenzel 1995), and hence the potential for aerobic processes such as CH4 oxidation (Grünfield and Brix 1999; Megonigal and Schlesinger 2002). Soils in low marsh areas that are permanently or frequently inundated experience low rates of O2 diffusion and sustain anaerobic environments where methanogenesis can occur (Ding et al. 2010). Tidal wetland studies have documented correlations between elevation, water level, and CH4 emissions (Grünfield and Brix 1999; Altor and Mitsch 2006; Ding et al., 2010; Audet et al. 2013), implicating hydroperiod as a dominant influence on wetland CH4 emissions.
Both plant community composition and water table depth have proven to be effective proxies for predicting CH4 emissions in non-tidal wetland ecosystems such as peatlands (Bubier et al. 1995; Couwenberg et al. 2011; Dias et al. 2010; Audet et al. 2013). Wetland plant species exhibit different tolerances to inundation (Sorrell et al. 2000; Vann and Megonigal 2003), leading to varying plant community composition across elevation gradients (Perry and Hershner 1999). Wetland vegetation is well suited for serving as a proxy to predict CH4 fluxes due to direct and indirect influences of plant species on labile soil organic carbon (i.e. root exudates), soil moisture, and CH4 gas transport via plant aerenchyma tissue (Couwenberg et al. 2011). Previous studies have established direct links between plant species composition and CH4 fluxes (Shäefer et al. 2011; Audet et al. 2013; Bhullar et al. 2014). Plant species composition has been established and implemented as a means to accurately predict CH4 fluxes from peatlands (Couwenberg et al. 2011; Dias et al. 2010), but this development has not yet occurred for tidal wetland systems. Water table depth, as influenced by relative elevation, has also proven to be a good proxy for predicting CH4 emissions in peatlands as water table level determines aerobic/anaerobic zones and redox states in the soil profile (Ding et al. 2010). Plant community composition, water table depth, and elevation co-vary in wetlands such that these parameters are often used as proxies for one another (Broome et al. 1995; Tuxen et al. 2011; Holmquist et al. 2021).
The objective of this study was to assess and advance our understanding of the potential of hydrology and plant species composition as proxies for CH4 emissions in brackish marshes at the site scale. We hypothesized that CH4 emissions would vary across strata defined by plant community composition and elevation in a tidal marsh that was relatively homogenous with respect to salinity. If such variation exists it suggests that a deeper process-level understanding of the biogeochemical consequences of plant–microbe-hydrology interactions can be widely applied to develop improved proxies to predict CH4 emissions in tidal wetlands. We measured CH4 fluxes in two brackish marshes on the Deal Island Peninsula on the Eastern Shore of Maryland, USA across four different strata defined by water level and plant community composition. We collected field data on elevation, water level, soil temperature, and soil pore water SO42−, sulfides, pH, and salinity and laboratory soil incubations using field-collected soil cores to assess potential CH4 production.
Methods
Study area
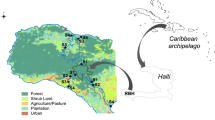
Our study area was located on the Deal Island Peninsula in Somerset County, Maryland, USA (38.185172 N, 75.906279 W) (Fig. 1). It consisted of two brackish tidal marshes, one unditched (Unditched) and one that had been ditched then restored (Ditched) located in the same marsh complex. Ditch plugs were installed at the Ditched site in April of 2014 by inserting a plastic polyethylene sheet vertically across the ditch approximately 50 m upstream from the tidal source and securing the plug using sediment sourced from the ditch upstream of the plug. The Ditched site had an overall lower elevation than the unditched site, and was primarily composed of Juncus roemerianus (black needlerush). The Unditched site had a more diverse species community including J. roemerianus, Spartina patens (salt marsh hay), Spartina alterniflora (smooth cordgrass), Phragmites australis (common reed), and Iva frutescens (marsh elder). Plant productivity in tidal marshes in this region includes a period of senescence during the late fall through the early spring, with peak plant productivity occurring in late July through August. Soils on site consist of thick moderately to highly decomposed organic horizons overlying loamy mineral horizons; within Soil Taxonomy they classify as the Mispillion series, Loamy, mixed, euic, mesic, Terric Sulfihemists, which are common estuarine marsh soils in this area. This microtidal marsh had a diurnal tidal range of approximately 0.6 m as measured in the adjacent tidal creek.
Study site and plot locations (inset) located on the Eastern Shore of Maryland, USA. S. alterniflora plots are marked with star icons, S. patens plots are marked with squares, High-elevation J. roemerianus plots are marked with diamonds, and Low-Elevation J. roemerianus plots are marked with circles
Design
Four strata that differed in their plant community composition and elevation, both of which are closely associated with water levels, were identified prior to the study from onsite observations and overhead aerial imagery. The strata corresponded to geographic units that may be used to estimate CH4 emissions when engaged in site-specific carbon crediting accounting methodologies (Emmer et al. 2015a, b). Water level variability was primarily controlled by elevation in these marshes, with lower elevations having higher water levels. Two of the strata had a plant community composition dominated by J. roemerianus, but differed in elevation; with one site at a “High” elevation and the other at a “Low” elevation. The High J. roemerianus stratum was located at the unditched site and had a mean elevation of 0.334 m relative to NAVD88, while the Low J. roemerianus stratum was located at the ditched site with a mean elevation 0.305 m. The two additional strata consisted of one dominated by S. alterniflora at a relatively low mean elevation of 0.299 m, and one dominated by S. patens at a relatively high mean elevation of 0.409 m. Both the Low S. alterniflora and High S. patens strata were located at the Unditched site. One representative area was selected within each stratum that included a range of elevation and plant diversity. A transect of five plots were established in each of the four strata for a total of 20 plots. Plots within a stratum were equally spaced across an area that we considered to be relatively homogenous with respect to plant community composition based on a visual assessment. Note that the objective of our study was to assess drivers of methane emissions rather than estimating emissions from the marsh complex, which would have required a larger number of randomly distributed sites across the full marsh area. Three of the strata were located within 25–50 m of a tidal creek, while one (S. alterniflora) was located in a more central location in the marsh complex approximately 100 m from a tidal creek. The 20 plots occupied an approximate area of 0.06 km2, with each 5-plot strata encompassing an approximate area of 1000 m2.
Field methods
We sampled monthly from April to December 2015; samples were not taken from January to March under the assumption that CH4 production would be negligible due to low temperatures (Marsh et al. 2005). Methane flux, air temperature, pore water pH, and pore water concentrations of SO42−, hydrogen sulfide, and CH4 were measured at each plot. Soil temperature at 10 cm was recorded at two plots per stratum hourly during the sampling season using HOBO 8 k Pendant sensors (Onset Corp., Bourne, MA). Soil temperature and water level data were not collected during the month of April because loggers were not ready for deployment until May.
Each of the 20 sample plots received a custom-fabricated square aluminum metal collar that was permanently inserted into the marsh to a depth of 10 cm nine months prior to the first sample. Flux chambers were constructed of an aluminum frame made of 2.5-cm wide angle stock covered with transparent polycarbonate plastic film. Chambers were placed on top of the collar about 10 min prior to sampling. Chambers were equipped with a closed-cell neoprene strip on the top and bottom, which when clamped to the collar assured an airtight seal (Yu et al. 2013). The taller plants in the J. roemerianus strata were accommodated without damaging plant stems by stacking chambers. Opaque chamber lids with a sampling port were clamped to the top of the chamber to complete the seal. Chambers had a height of 69.5 cm and an interior length and width of 49.5 cm, yielding a total volume of 0.17 m3 for single chambers and 0.34 m3 for double chambers. In order to prevent the weight of the observer from causing ebullition due to soil compression (Sorrell et al. 2013), each plot had a 3 m wooden boardwalk suspended above the soil surface by PVC legs for approaching the flux collar.
Methane flux samples were collected over a 1-h period from the 5 replicate flux plots in a given stratum. An initial sample was taken immediately after each chamber was sealed with four subsequent samples taken at approximately 15-min intervals for a total of 25 samples (5 per plot) over the 1-h period. Using a 30 mL syringe, the sampling port was opened and then expelled back into the chamber three to five times before each sample was taken. Each 18 mL air sample was withdrawn from the chamber and injected into a N2-flushed 12-mL Exetainer vial with rubber septum until analysis (Yu et al. 2013). Air temperature within the sampling chamber was recorded upon the collection of each flux sample from thermometers affixed to the interior of each chamber.
Porewater samples were taken at 10 cm depth using a porewater sipper and syringe (Fisher et al. 2013) and analyzed for pore water CH4, hydrogen sulfide (unfiltered), pH (unfiltered), salinity (unfiltered), and SO42− (filtered through a 0.45-µm filter) as described by Keller et al. (2009). Porewater CH4 was collected by withdrawing 15 mL of pore water, after which 15 mL of ambient air was drawn into the syringe and the syringe capped. The sample was then agitated for 1–2 min for the CH4 to be stripped into the drawn air, the stripped water was expelled, and the gas sample was stored in N2-flushed Exetainers for analysis (Keller et al. 2009). Hydrogen sulfide samples were diluted in a 1:1 ratio of sample to sulfide antioxidant buffer in the field to prevent sulfide volatilization and oxidation (Koch et al. 1990). Hydrogen sulfide and pH samples were analyzed the same day as sample collection; salinity was analyzed within two weeks in the laboratory using a YSI Model 3100 conductivity meter; and all other pore water samples were frozen and analyzed during the winter of 2016. We chose a sampling depth of 10 cm because it is within the root zone of the emergent species within the strata and close enough to the surface to be influenced by both aerobic and anaerobic processes in response to the fluctuating water table.
Additional data were collected during the July 2015 sampling event, which was predicted to be during a peak CH4 emission period. We collected porewater at 20 cm depth in addition to 10 cm and analyzed it for the same analytes excluding CH4 but including ferrous iron (Fe2+). We measured at 20 cm during this period of predicted high emissions to understand processes at a depth that is more dominated by anaerobic conditions.
Water level was measured at each stratum in order to determine water levels at the time of sampling and antecedent water level conditions during the two-week period leading up to the sampling period. Water level recorders (HOBO U20-L, Onset Corp, Bourne, MA) were installed adjacent to the chamber transects to continuously record water levels in the marsh; one was also installed in the tidal creek adjacent to the field site during the field season. We deployed two water level loggers in each stratum, except for the low water table J. roemerianus stratum, which had one water level logger due to its small area relative to the other strata. Barometric pressure was collected onsite to correct the unvented loggers (HOBO U20-L, Onset Corp, Bourne, MA). We surveyed the elevation of all 20 plots and water level logger locations using a Real-time Networking Global Positioning System (RTN GPS) unit, which provides elevation data with approximately 2 cm accuracy (http://www.keynetgps.com).
Soil cores were collected during the July sampling event and analyzed for potential anaerobic CH4 and CO2 production. Cores were collected from approximately 0–40 cm depth using a circular metal gouge corer with a diameter of 5.1 cm. The corer was inserted into the marsh, with careful attention paid to minimize compaction of the soft peat. The core was removed and cut at a depth of 20 cm, yielding two depth increments per plot. Cores were placed into sample bags in which as much air as possible was removed. The cores were then placed in a cooler with ice and transported back to the laboratory, where each bag was flushed three times with nitrogen gas to remove oxygen, stored on ice during transport, and placed in a 4 °C cold room until processing. Water for these incubations was collected from the bore hole from which the core was removed, stored on ice for transport back to the lab, purged with nitrogen gas to remove oxygen before being sealed and placed in a cold room at 4 °C. Soil cores and water samples were stored in the cold room within 8 h of their collection and incubated within 5 days.
Laboratory analyses
Flux chamber headspace samples were measured on a Varian 450 gas chromatograph using a Combi-Pal autosampler and corrected for dilution of 18 mL of sample into 12 mL of N2 in the Exetainer. Flux rate was calculated as the linear increase in headspace [CH4] over time based on measurements of chamber temperature and volume and assuming atmospheric pressure (n = 147 fluxes). The linear slope was calculated in Excel using the Regression function. Data points were excluded from the regression if they indicated an ebullition event (large spike in [CH4]) or an Exetainer leak (large drop in [CH4]). Fluxes were calculated from five points (n = 79), four points (n = 61), but never from fewer than three points (n = 23). No flux measurements were excluded based on arbitrary regression R2 or p value limits to prevent introducing bias against low fluxes that approach the detection limits of our experimental system (Pitz and Megonigal 2017). However, fluxes were excluded in several cases where an ebullition event or leak was large compared to the CH4 flux rate (n = 17). In cases where there was no significant trend in headspace [CH4] and no evidence of ebullition or leaks the flux was assigned a value of zero (n = 16). Because most of the excluded fluxes were collected during periods of low CH4 flux, they had relatively little influence on the annual flux calculation.
To estimate annual emissions we averaged rates from each measurement campaign. We assumed that the fluxes in the unsampled months of January, February, and March were equal to our observed values from April. The twelve monthly values were averaged and converted to annual units. While this method likely overestimated CH4 emissions, overestimation is the conservative and therefore preferable approach for carbon credit accounting (Needelman et al. 2018).
Hydrogen sulfide was determined with a Lazar Laboratory model 146S sulfide electrode. Sulfide antioxidant buffer was prepared the day before sample collection with deoxygenated (N2-stripped) distilled water, sodium salicylate, sodium hydroxide, and ascorbic acid according to Koch et al. (1990). A standard curve created from a serial dilution of a Na2S/buffer solution prepared on the day of each sampling event and readings were complete within 4 h of sample collection. The pH was measured with a YSI Pro Plus (https://www.ysi.com) pH meter calibrated with standards at pH 7.0 and 10.0. Salinity was measured with a calibrated conductivity/salinity electrode. The remaining analytes SO42− and Fe2+ were quantified at the Chesapeake Biological Laboratory, Solomons Island, MD. Reduced iron was analyzed according to EPA method 200.1 and SO42− was analyzed according to the National Environmental Methods Index Standard Methods: 4110B for ions in water by ion chromatography (www.nemi.gov).
Sulfate depletion was calculated by assuming the SO42− concentration in the absence of sulfate reduction was that of full-strength marine seawater (Canfield 2004) diluted to the observed salinity of our porewater sample. We then divided our observed SO42− concentration by this expected concentration of SO42− to estimate the consumption of SO42− (i.e. relative depletion) in the porewater.
Soil cores collected for incubations were removed from cold storage within 5 days of collection and placed into an anaerobic hood containing a N2/H2 mixture (Megonigal and Schlesinger 2002). Two sections were removed from each core, yielding a 8–12 cm depth sample and a 28–32 cm depth sample. The outer 10 mm (approximately) of the resulting disks were removed to expose the center of the core, which was assumed to have had minimal O2 exposure from collection to processing. We then removed as many live roots as feasible. Five grams of wet soil material was placed in a 35-mL serum bottle with 5 mL of the degassed water from the core hole. Headspace samples of 0.5 mL were taken daily for approximately two weeks, and were injected directly into a Shimadzu gas chromatograph with a flame ionization detector for CH4 or a LI-COR LI-7000 (LiCor, Lincoln, NE) for CO2. Methanogenesis generally slowed dramatically after 5 days, so our calculations of potential anaerobic CH4 production rates are based on incubation days 1–5.
Statistical analysis
Data were analyzed using SAS 9.3 (SAS Institute Inc. Cary, NC). Regression analyses were performed on flux data using Proc Reg to determine the slope of CH4 or CO2 concentration change over time. All variables were evaluated for normality using PROC UNIVARIATE and those that required transformation were log transformed to improve normality. Parameters transformed were: CH4 flux, pore water hydrogen sulfide concentration, pore water SO42− concentration, and pore water CH4 concentrations. All parameters were analyzed using PROC MIXED with strata and month in the model statement with repeated measures. Post-hoc Tukey mean comparisons were used with α = 0.05 used to indicate significance.
Results
Antecedent water levels
Water level data collected during the 2 weeks prior to and during sampling events varied significantly between strata (p < 0.0001) and month (p < 0.0001). Water levels of the S. patens stratum were significantly lower than all other strata, with a mean of 9 cm below the soil surface, while the other strata had similar mean water levels of approximately 1 cm below the soil surface (Table 1). We were unable to test for a strata by month interactive effect because only two wells were deployed in each strata (and only one in the High J. roemerianus stratum); however, S. patens had a lower mean water level in all months (Derby 2016). Mean water levels were highest in July, August, and October and lowest in May, June, September, and December.
Methane emissions and porewater chemistry by strata
Average CH4 flux over the study varied significantly between strata (p < 0.0001) and month (p < 0.0001), and had a significant interactive effect (p = 0.018). Methane emissions from the four strata ranked S. alterniflora ≫ High J. roemerianus > Low J. roemerianus = S. patens (Table 1). Mean CH4 emissions from the S. alterniflora stratum was 2.72 times greater than the next highest CH4 emitter (Table 1) despite similar inundation regimes among the three highest emitting strata. High CH4 emissions in the S. alterniflora stratum coincided with significantly higher porewater CH4 concentrations and lower SO42− concentrations than the other three strata (Table 1). Porewater salinity in the S. alterniflora stratum was similar to other strata (Table 1) suggesting that relatively low SO42− concentrations were due to high rates of SO42− consumption. Indeed, SO42− was depleted by 61% in the S. alterniflora stratum. Sulfate depletion was significantly different between strata (p < 0.0001) and month (p < 0.0001) and ranked S. alterniflora = High J. roemerianus > Low J. roemerianus > S. patens. Sulfate depletion rates in the S. alterniflora stratum were over two times greater than those seen in low J. roemerianus (Table 1). Low SO42− concentrations in the S. alterniflora stratum were accompanied by significantly higher amounts of hydrogen sulfide (72 mg L−1) as compared to the other three strata (all < 20 mg L−1).
The S. patens stratum had the lowest mean porewater CH4 concentrations and SO42− depletion rates, with only 1.4% SO42− depleted. S. patens also exhibited the highest mean concentrations of reduced iron (i.e. ferrous iron, Fe2+) during the single campaign when it was measured, with 72 mg L−1 of ferrous iron in porewater collected 10 cm below the soil surface (Table 1). None of the other three strata had reduced iron porewater concentrations exceeding 0.8 mg L−1. S. patens also had a significantly lower mean porewater salinity (12.3 ppt) than the other three strata, which ranged in mean salinity from 14.2 to 14.8 ppt. The High and Low J. roemerianus and S. alterniflora strata were not significantly different in porewater iron concentrations or salinity (Table 1).
The difference in elevation between the two J. roemerianus strata was not highly apparent in the water level and CH4-related attributes we measured. The two strata were not significantly different from one another in salinity, SO42− concentrations, sulfide concentrations, percent SO42− depleted, porewater CH4 concentrations, and reduced iron concentrations (Table 1).
The highest CH4 emissions were observed in July, August, and September; the lowest were observed in April, November, and December (Fig. 2). Significant strata by month interactions were observed in May, June, and September. In May and June, S. alterniflora was not significantly different from any strata other than Low J. roemerianus; all other strata were not significantly different from one another. In September, S. alterniflora was not significantly different from any strata other than S. patens; all other strata were not different from one another. No significant within-month differences were observed in April, July, August, October, November and December (Derby 2016). Porewater CH4 exhibited a similar seasonal trend as CH4 emissions, with the highest concentrations in the months July through November (Derby 2016).
Mean hourly methane flux during the sampling period in a tidal brackish marsh on the Deal Island Peninsula on Maryland’s Eastern Shore. “Low” vs “High” refers to comparative elevations within the site. Error bars signify standard error; significant differences are shown with letters. ANOVA results of the log transformed data showed significant differences between strata (p < 0.0001), month (p < 0.0001) and strata*month (p = 0.0182)
Anaerobic incubations
Surficial soils (8–12 cm) from the S. patens stratum had the lowest CH4 production, highest CO2 production and a significantly higher ratio of CO2:CH4 production (ratio = 993) that the other strata. At the other extreme was the S. alterniflora stratum which produced substantially more CH4 and less CO2 than the other strata, and therefore had the lowest CO2:CH4 ratio (ratio = 40) among the four sites (Table 2). The two J. romerianus strata fell in between these extremes with a CO2:CH4 ratio of about 200 (Table 2), though there were no significant differences in CO2:CH4 ratio between these strata and S. alterniflora. The 10 cm incubations produced significantly more CH4 and CO2 than those from 30 cm depth (p = 0.04, data not shown, Derby 2016).
Discussion
Methane emissions varied by plant community type and hydrologic setting, suggesting that these variables have potential to be developed into cost-effective proxies to estimate CH4 emissions from tidal marshes. Mean emissions across strata ranked as S. alterniflora > High-elevation J. roemerianus > Low-elevation J. roemerianus > S. patens (Table 1). The mechanisms that led to these differences among strata need to be understood to develop vegetation- and hydrology-based proxies, as their application is likely to be more complex than the salinity proxity.
We attribute the low emissions from the S. patens stratum to a combination of relatively low water levels, shallow rooting depth, and higher mineral inputs, all of which have the capacity to suppress CH4 production and promote CH4 oxidation. It is well established that CH4 production is suppressed by alternative electron acceptors such as O2, Fe(III) and SO42− (Roden and Wetzel 2003; Neubauer et al. 2005; Poffenbarger et al 2011; Holm et al. 2016). Relatively high O2 flux into the upper soil surface (0–10 cm depth) would be favored by both the relatively thick aerobic zone (i.e. deeper water-table) and shallow distribution of root biomass that is characteristic of S. patens communities (Bernal et al. 2016). Mean antecedent water depth was 9 cm deep in this stratum compared to other strata with water levels near the soil surface.
Because the majority of S. patens roots are in the top 10 cm of the soil profile (Windham 2001), it is likely that root oxygen loss was also an O2 source in this community. O2 suppresses methanogenesis via electron donor competition by two mechanisms, directly as an electron acceptor for aerobic bacteria and indirectly by regenerating poorly crystalline iron oxides on the root surface (i.e. iron plaque). Root-deposited iron plaque is rapidly consumed by iron-reducing bacteria (Weiss et al. 2003, 2004), suppressing both SO42− reduction and methanogenesis (Neubauer et al. 2005). This mechanism is consistent with our observation that the S. patens community had dramatically higher concentrations of reduced iron (measured only in July) and SO42− than the other strata, and the least SO42− depletion (Table 1). The close proximity of this site to the tidal creek may have also allowed for greater mineral inputs during flooding events to support iron cycling. Finally, if rates of methanotrophy are O2-limited as studies suggest (King 1996; Lombardi et al. 1997; Megonigal and Schlesinger 2002), then relatively high rates of CH4 oxidation would be expected to further decrease the amount of CH4 emitted through passive diffusion through plants, as documented in other tidal wetlands (Megonigal and Schlesinger 2002).
The S. alterniflora stratum had the highest average CH4 emissions and porewater chemistry that differed from the S. patens community in several respects (Table 1). The S. alterniflora stratum was in the center of the marsh complex (Fig. 1). Because hydrologic fluxes generally decrease with increasing distance from the tidal creek (Jordan et al. 1985), it is likely that soils in the S. alterniflora stratum had relatively slow rates of advection compared to the other strata. Indeed, water table depths decreased relatively slowly after floods in the S. alterniflora stratum (Derby 2016). This hydrologic difference likely decreased rates of advective transport of O2 and SO42− to the soil profile to replenish these electron acceptors. We propose that the relatively low inputs of SO42− to the S. alterniflora stratum led to SO42− limitation of SO42− reduction rates, allowing methanogens to compete more effectively with sulfate-reducing bacteria for electron donors. Porewater evidence supporting this interpretation includes low concentrations of Fe2+, high SO42− depletion, and high concentrations of both hydrogen sulfide and CH4. This interpretation is also consistent with the results of the July anaerobic incubations showing that the CO2:CH4 ratio was lowest in S. alterniflora soils and highest in S. patens soils. Because aerobic respiration, sulfate reduction, and iron reduction generate CO2 rather than CH4, these data suggest that methanogens had relatively little competition for organic carbon and H2 in the S. alterniflora stratum.
Water table depths in the High and Low J. roemerianus strata were similar to the S. alterniflora stratum but metrics related to CH4 emissions were consistently lower than the S. alterniflora and higher than the S. patens strata, namely CH4 emissions, porewater SO42− concentrations, and anaerobic incubation CO2:CH4 ratio. The difference in CH4 emissions between S. alterniflora and the J. roemerianus strata cannot be explained by water levels, salinity, pH, or reduced iron because they were not significantly different between these strata. For example, mean water table depth in the S. alterniflora stratum and the two J. roemerianus strata were within 1 cm of each other, while emissions were 2.5 times greater in the S. alterniflora stratum. The J. roemerianus strata were closer to the tidal creek and presumably CH4 production was not limited by tidal inputs of SO42−supply as we suspect was the case in the S. alterniflora stratum. However, the relatively low CH4 emissions in the J. roemerianus strata compared to S. alterniflora may also have been related to plant traits that regulate CH4 emissions by influencing the balance between CH4 production and oxidation (Sutton-Grier and Megonigal 2011; Mueller et al. 2020), which itself is influenced by traits that affect CH4 transport through plants (Komiya et al. 2020). Mueller et al. (2020) proposed that plant traits vary across species such that some push the balance between these opposing processes toward net CH4 production while others favor net CH4 oxidation. We hypothesize that among the dominant species present at the site, S. alterniflora favors net CH4 production while J. roemerianus favors net CH4 oxidation, and that the lower CH4 emissions rates in the J. roemerianus strata may have been due to relatively high rates of root oxygen loss by J. roemerianus. This interpretation is supported in part by data on porewater [SO42−], which mediates the outcome of competition between sulfate-reducing bacteria and methanogens. Porewater [SO42−] was 4.5 mM in the S. alterniflora stratum but exceeded 6 mM in the J. roemerianus strata where CH4 emissions rates were relatively low. Although this difference in porewater [SO42−] seems small, the relationship between these variables is non-linear and displays a threshold value above which sulfate reduction dominates and below which methanogenesis dominates (Megonigal et al. 2004). There is a general lack of CH4-relevant porewater data for coastal wetlands, but a robust record from a brackish marsh located 100 km from the study site in Chesapeake Bay observed that porewater [CH4] declined abruptly when SO42− concentrations exceeded approximately 4 to 6 mM (Keller et al. 2009). We propose that the S. alterniflora and J. roemerianus strata fell on opposite sides of this threshold.
Although floodwater salinity can be an effective proxy for porewater SO42− concentrations when comparing sites across large spatial scales (Poffenbarger et al. 2011), our data demonstrate the limitations of using the salinity proxy at local scales. Variation among strata in SO42− concentration and SO42− depletion may have been caused by variation in rates of SO42− transport from tidal floodwater into soils, sulfide oxidation related to O2 diffusion from water table depth or root O2 loss, or primary production (i.e. carbon availability). We cannot distinguish among these mechanisms with the present dataset. Sulfate depletion was a better indicator of CH4 flux than salinity or SO42− concentration alone and may prove to be a superior proxy for CH4 emissions in tidal brackish marshes (Keller et al. 2009; Noyce and Megonigal 2021).
Within our strata, CH4 production did not strictly follow the conventional interpretation that differences in the free energy yield among competing microbial respiration processes means that just one process dominates microbial respiration at a time, with methanogenesis expected to occur only when all other electron acceptors are fully (or nearly) depleted. Our data suggest that peak sulfate reduction activity was occurring concurrently with peak CH4 production in the S. alterniflora stratum which had both the highest mean pore water hydrogen sulfide concentrations and the lowest SO42− concentrations. We attribute this to spatial variation in electron donors and acceptors that creates microsites of high SO42− depletion and methanogenesis. Microsites have been shown to produce small amounts of CH4 in upland forested systems, originally thought to be too dry and too aerobic to produce this greenhouse gas (Megonigal and Guenther 2008). Microsites also exist in tidal marsh soils due to local (i.e. rhizosphere) consumption of electron acceptors at rates faster than they can be replenished (e.g. Rabenhorst et al. 2010).
Carbon offset implications
Salinity is a useful proxy for CH4 emissions from tidal marshes with salinity regimes > 18 ppt because CH4 emissions are low in most systems compared to their soil carbon sequestration rates and variation among marshes is small (Poffenbarger et al. 2011). However, tidal brackish marshes at lower salinities may emit enough CH4 to offset a significant fraction of their radiative cooling and variation in CH4 emissions among marshes within a given salinity regime is large. Such uncertainty is accommodated in carbon offset programs such as the Verified Carbon Standard by requiring the project to estimate CH4 emissions by direct monitoring or by using published data, a model, or a proxy that can be demonstrated to be valid for the project site (Needelman et al. 2018). Stratification by hydrology and vegetation characteristics may provide a more effective proxy than salinity to estimate CH4 emissions.
We compared the CH4 offset estimates produced through our direct measurements to those predicted by the salinity-based proxy equation of Poffenbarger et al. (2011). For this comparison we assumed a single soil carbon sequestration rate of 1.46 Mg C ha−1 year−1 for all strata in the marsh complex, which is the default rate in the carbon crediting methodology of Emmer et al. (2015a, b), derived as the median value from Chmura et al. (2003). In three of the four strata the salinity proxy overestimated emissions by about 20%, 40%, and 80% (Table 3). Overestimation is preferable to underestimation to avoid awarding carbon credits that exceed actual greenhouse gas benefits, but overestimation decreases the financial viability of carbon offset projects. These errors caused the positive radiative balance to be underestimated by just 4–8% in the J. roemerianus strata, suggesting that incorporating vegetation and hydrology proxies would not be a substantial improvement over the salinity proxy alone in these strata. However, the positive radiative balance of the S. patens stratum was underestimated by > 20%, suggesting that a proxy based on vegetation and hydrology would improve CH4 emission estimates. The salinity proxy overestimated CH4 emissions in the S. alterniflora statum where the positive radiative balance was overestimated by about 25% (Table 3), indicating that a salinity-based proxy would award carbon credits exceeding actual climate benefits in this stratum. Improved proxies are needed to incentivise carbon offsets projects by reducing monitoring costs while ensuring that projects achieve estimated climate benefits.
Our results suggest that proxies for CH4 emissions from tidal wetlands with salinity regimes < 18 ppt can be improved by incorporating metrics related to hydrology such as flooding frequency and duration or the position of the soil surface relative to the tidal frame, and metrics related to plant traits such as species identity, plant functional type, or biomass. Ideally these metrics would be identifiable at high spatial resolution for low cost, such as through analysis of freely available remote sensing data. Currently there is no widely accepted method to remotely sense surface water salinity, but robust methods exist for remote sensing of plant cover, biomass, primary production, and elevation (Buffington et al. 2016; Byrd et al. 2018; Feagin et al. 2020).
Direct monitoring is not economically feasible for many carbon crediting projects due to the high spatial and temporal variability of methane emissions (Needelman et al. 2018). For example, the Verified Carbon Standard requires a sufficient sample size to achieve a targeted confidence interval of 95% with 30% allowable error, otherwise a uncertainty deduction must be taken from carbon credits earned. The required sample size for a given project is a function of the coefficient of variation. The coefficients of variation in our study at peak methane emissions (July) ranged from 0.4 in the S. patens stratum to 1.1 in the S. alterniflora stratum. To meet this sample size requirement in the S. alterniflora stratum, 56 sampling plots would be required. Further, these points would need to be randomly distributed throughout the strata, further increasing project cost. Due to the high costs of direct monitoring, alternative methods to estimate methane emissions are required such as proxies and models. Under the Verified Carbon Standard, projects seeking to apply these alternative methods must demonstrate that the method was developed or validated in a comparable system as the project area with similar geomorphic, hydrologic, and biological properties and similar management regimes, unless the project can argue that any differences should not have a substantial effect on methane emissions (Emmer et al. 2020a). For this reason, the development and application of vegetation- and hydrology-based proxies will require a deeper process-level understanding of the geomorphic, hydrologic, biological, and management-related drivers of methane emissions.
Conclusions
Tidal wetland restoration and conservation projects have the potential to mitigate greenhouse gas emissions to the atmosphere and generate carbon credits, but due to the high cost of direct monitoring, a better understanding of the factors influencing wetland CH4 emissions in brackish and freshwater systems (salinity < 18 ppt) is required to develop cost-efficient proxies to estimate CH4 at site-specific scales. We found significantly different methane emission rates across four strata defined by hydrology and plant community composition that otherwise had similar salinity regimes. We inferred that they deviated from the rates predicted by a salinity proxy due to processes that regulate the availability of competing terminal electron acceptors such as O2, Fe(III), and SO42− as mediated by plant traits that regulate CH4 emissions. Low CH4 emission rates in the high-elevation S. patens stratum was attributed to relatively high availability of electron acceptors such as O2, Fe(III), and SO42− through tidal inputs and O2 diffusion across soil and root surfaces. Oxygen is particularly important because it suppresses CH4 emissions by three mechanisms: aerobes competing for electron donor compounds are favored over methanogens; O2 regenerates oxidized forms Fe and S that support more competitive forms of anaerobic respiration; and O2 supports CH4 oxidation. By contrast, the S. alterniflora stratum was hydrologically isolated from tidal inputs of Fe(III) and SO4 and supported relatively little O2-driven regeneration of these compounds due to a high water table. This site was the most depleted in SO42− and presumably relatively depleted in O2 and Fe(III) as well, thereby supporting high rates of methanogenesis. The greater CH4 emissions from the low-elevation S. alterniflora stratum than the low and high elevation J. roemerianus strata could not be explained by water table depth, salinity, pH, and [Fe2+], suggesting an important role for plant traits such as root O2 loss regulating CH4 emissions at local scales. The mechanisms driving these patterns were not measured directly but likely involve variations in rates of SO42− diffusion from tidal floodwater based on site hydrologic connectivity; rates of sulfate regeneration via sulfide oxidation as influenced by O2 diffusion or root O2 loss; and differing plant primary productivity.
Our results suggest that vegetation and hydrology can be effective proxies to estimate CH4 emissions from tidal marshes. Our study contributes to the literature of field studies testing for differences in emissions between strata differentiated by vegetation and/or hydrology. The development and application of vegetation- and hydrology-based proxies is expected to be more complicated than the salinity proxy, necessitating an improved process-level understanding of the biogeochemical consequences of plant–microbe-hydrology interactions. We included extensive covariates in our sampling design, thereby elucidating insights into the mechanisms by which these potential proxies drive CH4 emissions, which we hope will spur further development of these proxies and promote research on key associated biogeochemical processes.
Data availability (data transparency)
All data from this manuscript will be made available through the Coastal Carbon Research Coordination Network (https://doi.org/10.25573/serc.14227085).
References
Al-Haj AN, Fulweiler RW (2020) A synthesis of methane emissions from shallow vegetated coastal ecosystems. Glob Change Biol 26:2988–3005. https://doi.org/10.1111/gcb.15046
Altor AE, Mitsch WJ (2006) Methane flux from created riparian marshes: relationship to intermittent versus continuous inundation and emergent macrophytes. Ecol Eng 28(3):224–234. https://doi.org/10.1016/j.ecoleng.2006.06.006
Audet J, Johansen JR, Andersen PM, Baattrup-Pedersen A, Brask-Jensen KM, Elsgaard L, Hoffmann CC (2013) Methane emissions in Danish riparian wetlands: ecosystem comparison and pursuit of vegetation indexes as predictive tools. Ecol Ind 34:548–559. https://doi.org/10.1016/j.ecolind.2013.06.016
Bernal B, Megonigal JP, Mozdzer TJ (2016) An invasive wetland grass primes deep soil carbon pools. Glob Change Biol 23(5):2104–2116. https://doi.org/10.1111/gcb.13539
Bhullar GS, Edwards PJ, Venterink HO (2014) Influence of different plant species on methane emissions from soil in a restored swiss Wetland. PLoS ONE. https://doi.org/10.1371/journal.pone.0089588
Broome SW, Mendelssohn IA, McKee KL (1995) Relative growth of Spartina patens (Ait.) Muhl. and Scirpus olneyi gray occurring in a mixed stand as affected by salinity and flooding depth. Wetlands 15:20–30. https://doi.org/10.1007/BF03160676
Bubier JL, Moore TR, Bellisario L, Comer NT, Crill PM (1995) Ecological controls on methane emissions from a Northern Peatland Complex in the zone of discontinuous permafrost, Manitoba Canada. Global Biogeochem Cycle 9(4):455–470. https://doi.org/10.1029/95gb02379
Buffington KJ, Dugger BD, Thorne KM, Takekawa JY (2016) Statistical correction of lidar-derived digital elevation models with multispectral airborne imagery in tidal marshes. Remote Sens Environ 186:616–625
Byrd KB, Ballanti L, Thomas N, Nguyen D, Holmquist JR, Simard M, Windham-Myers L (2018) A remote sensing-based model of tidal marsh aboveground carbon stocks for the conterminous United States. ISPRS J Photogramm Remote Sens 139:255–271
Calhoun A, King GM (1997) Regulation of root-associated methanotrophy by oxygen availability in the rhizosphere of two aquatic macrophytes. Appl Environ Microbiol 63(8):3051–3058. https://doi.org/10.1128/aem.63.8.3051-3058.1997
Canfield DE (2004) The evolution of the earth surface sulfur reservoir. Am J Sci 304:839–861. https://doi.org/10.2475/ajs.304.10.839
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Glob Biogeochem Cycles 17:1111. https://doi.org/10.1029/2002GB001917
Colmer TD (2003) Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant, Cell Environ 26(1):17–36. https://doi.org/10.1046/j.1365-3040.2003.00846.x
Couwenberg J, Thiele A, Tanneberger F, Augustin J, Bärisch S, Dubovik D, Liashchynskaya N, Michaelis D, Minke M, Skuratovich A, Joosten H (2011) Assessing greenhouse gas emissions from peatlands using vegetation as a proxy. Hydrobiologia 674(1):67–89. https://doi.org/10.1007/s10750-011-0729-x
Crooks S, Herr D, Tamelander J, et al (2011) Mitigating climate change through restoration and management of coastal wetlands and near-shore marine ecosystems: challenges and opportunities.
Derby, K (2016) Methane emissions from a tidal brackish marsh on Maryland’s eastern shore and the factors impacting them. Master’s Thesis, University of Maryland. Drum https://doi.org/10.13016/M2PV6R
Dias AT, Hoorens B, Logtestijn RS, Vermaat JE, Aerts R (2010) Plant species composition can be used as a proxy to predict methane emissions in peatland ecosystems after land-use changes. Ecosystems 13(4):526–538. https://doi.org/10.1007/s10021-010-9338-1
Ding W, Cai Z, Tsuruta H (2005) Plant species effects on methane emissions from freshwater marshes. Atmos Environ 39(18):3199–3207. https://doi.org/10.1016/j.atmosenv.2005.02.022
Ding W, Zhang Y, Cai Z (2010) Impact of permanent inundation on methane emissions from a Spartina alterniflora coastal salt marsh. Atmos Environ 44(32):3894–3900. https://doi.org/10.1016/j.atmosenv.2010.07.025
Emery H, Fulweiler W (2014) Spartina alterniflora and invasive Phragmites australis stands have similar greenhouse gas emissions in a New England marsh. Aquat Bot. https://doi.org/10.1016/j.aquabot.2014.01.010
Emery HE, Fulweiler RW (2017) Incomplete tidal restoration may lead to persistent high CH4 emission. Ecosphere 8(12):e01968. https://doi.org/10.1002/ecs2.1968
Emmer IM, Unger M von, Needelman B, Crooks, S, Emmett-Mattox, S (2015) Coastal Blue Carbon in Practice: A Manual for Using the VCS Methodology for Tidal Wetland and Seagrass Restoration. Restore America's Estuaries, Arlington, VA., v. 1, p 82. https://www.estuaries.org/images/rae_coastal_blue_carbon_methodology_web.pdf
Emmer IM, von Unger MBA, Cooks NS, S. Emmett-Mattox. 2015. Coastal blue carbon in practice: a manual for using the vcs methodology for tidal Wetland and Seagrass Restoration
Emmer IM, Needelman BA, Emmett-Mattox S, Crooks S, Megonigal JP, Myers D, Oreska MPJ, McGlathery KJ (2020a) Estimation of baseline carbon stock changes and greenhouse gas emissions in Tidal Wetland restoration and conservation Project Activities (BL-TW). VCS Module VMD0050, v 1.0. Verra (Verified Carbon Standard): Washington, D.C
Emmer IM, Needelman BA, Emmett-Mattox S, Crooks S, Megonigal JP, Myers D, Oreska MPJ, McGlathery KJ (2020b) Methods for monitoring of carbon stock changes and greenhouse gas emissions and removals in Tidal Wetland restoration and conservation project activities (M-TW). VCS Module VMD0051, v 1.0, Verra (Verified Carbon Standard): Washington, DC
Epp MA, Chanton JP (1993) Rhizospheric methane oxidation determined via the methyl fluoride inhibition technique. J Geophys Res Atmos 98(D10):18413–18422. https://doi.org/10.1029/93jd01667
Feagin RA, Forbrich I, Huff TP, Barr JG, Ruiz‐Plancarte J, Fuentes JD, Najjar RG, Vargas R, Vázquez‐Lule A, Windham‐Myers L, Kroeger KD, Ward EJ, Moore GW, Leclerc M, Krauss KW, Stagg CL, Alber M, Knox SH, Schäfer KVR, Bianchi TS, Hutchings JA, Nahrawi H, Noormets A, Mitra B, Jaimes A, Hinson AL, Bergamaschi B, King JS, Miao G (2020) Tidal Wetland gross primary production across the continental United States, 2000–2019. Global Biogeochemical Cycles 34
Fisher MM, Reddy KR, DeLaune RD et al (2013) Soil pore water sampling methods. In: DeLaune RD, Reddy KR, Richardson CJ, Megonigal JP (eds) Methods in biogeochemistry of wetlands, vol 10, SSSA Book Series. Soil Science Society of America, Madison, WI
Garcia J-L, Patel BKC, Ollivier B (2000) Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe 6:205–226. https://doi.org/10.1006/anae.2000.0345
Gilbert B, Frenzel P (1995) Methanotrophic bacteria in the rhizosphere of rice microcosms and their effect on porewater methane concentration and methane emission. Biol Fertil Soils 20(2):93–100. https://doi.org/10.1007/bf00336586
Grünfeld S, Brix H (1999) Methanogenesis and methane emissions: effects of water table, substrate type and presence of Phragmites australis. Aquat Bot 64(1):63–75. https://doi.org/10.1016/s0304-3770(99)00010-8
Holm GO, Perez BC, McWhorter DE et al (2016) Ecosystem level methane fluxes from tidal freshwater and brackish marshes of the mississippi river delta: implications for coastal wetland carbon projects. Wetlands. https://doi.org/10.1007/s13157-016-0746-7
Holmquist J, Windham-Myers L, Bernal B, Byrd KB, Crooks S, Gonneea ME, Herold N, Knox SH, Kroeger K, McCombs J, Megonigal JP, Meng L, Morris JT, Sutton-Grier AE, Troxler TG, Weller D (2018) Uncertainty in United States coastal wetland greenhouse gas inventorying. Environ Res Lett 13:115005. https://doi.org/10.1088/1748-9326/aae157
Holmquist JR, Schile-Beers L, Buffington K, Lu M et al (2021) Scalability and performance tradeoffs in quantifying relationships between elevation and tidal wetland plant communities. Mar Ecol Prog Ser 666:57–72. https://doi.org/10.3354/meps13683
Jespersen DN, Sorrell BK, Brix H (1998) Growth and root oxygen release by Typha latifolia and its effects on sediment methanogenesis. Aquat Bot 61(13):165–180. https://doi.org/10.1016/s0304-3770(98)00071-0
Jordan TE, Correll ADL (1985) Nutrient chemistry and hydrology of interstitial water in brackish tidal marshes of Chesapeake Bay. Estuar Coast Shelf Sci 21(1):45–55
Keller JK, Wolf AA, Weisenhorn PB et al (2009) Elevated CO2 affects porewater chemistry in a brackish marsh. Biogeochemistry 96:101–117. https://doi.org/10.1007/s10533-009-9347-3
King GM (1996) In situ analyses of methane oxidation associated with the roots and rhizomes of a bur reed, sparganium eurycarpum, in a Maine Wetland. Appl Environ Microbiol 62:4548–4555. https://doi.org/10.1128/aem.62.12.4548-4555.1996
Koch MS, Mendelssohn IA, McKee KL (1990) Mechanism for the hydrogen sulfide- induced growth limitation in wetland macrophytes. Limnol Oceanogr 35:399–408. https://doi.org/10.4319/lo.1990.35.2.0399
Koebsch F, Glatzel S, Jurasinski G (2013) Vegetation controls methane emissions in a coastal brackish fen. Wetl Ecol Manag 21:323–337. https://doi.org/10.1007/s11273-013-9304-8
Komiya S, Yazaki T, Kondo F, Katano K, Lavric JV, Mctaggart I, Pakoktom T, Siangliw M, Toojinda T, Noborio K (2020) Stable carbon isotope studies of CH4 dynamics via water and plant pathways in a tropical thai paddy: insights into diel CH 4 transportation. J Geophys Res Biogeosci 125:9. https://doi.org/10.1029/2019jg005112
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37(1):25–50. https://doi.org/10.1016/s1164-5563(01)01067-6
Lombardi AE, Epp MA, Chanton JP (1997) Investigation of the methyl fluoride technique for determining rhizospheric methane oxidation. Biogeochemistry 36:153–172
Marsh AS, Rasse DP, Drake BG, Megonigal JP (2005) Effect of elevated CO2 on carbon pools and fluxes in a brackish marsh. Estuaries 28:694–704
Megonigal JP, Guenther AB (2008) Methane emissions from upland forest soils and vegetation. Tree Physiol 28:491–498. https://doi.org/10.1093/treephys/28.4.491
Megonigal JP, Hines ME, Visscher PT (2004) Anaerobic metabolism: linkages to trace gases and aerobic processes. In: Schlesinger WH (ed) Biogeochemistry. Elsevier-Pergamon, Oxford, pp 317–424
Megonigal JP, Schlesinger WH (2002) Methane-limited methanotrophy in tidal freshwater swamps. Glob Biogeochem Cycles 16:1088. https://doi.org/10.1029/2001GB001594
Moor H, Rydin H, Hylander K, Nilsson MB, Lindborg R, Norberg J (2017) Towards a trait-based ecology of wetland vegetation. J Ecol 105:1623–1635. https://doi.org/10.1111/1365-2745.1273
Mueller P, Hager RN, Meschter JE, Mozdzer TJ, Langley JA, Jensen K, Megonigal JP (2016) Complex invader-ecosystem interactions and seasonality mediate the impact of non-native Phragmites on CH4 emissions. Biol Invasions 18:2635–2647. https://doi.org/10.1007/s10530-016-1093-6
Mueller P, Mozdzer TJ, Langley JA, Aoki LR, Noyce GL, Megonigal JP (2020) Plant species determine tidal wetland CH4 response to sea level rise. Nat Commun. https://doi.org/10.1038/s41467-020-18763-4
Nahlik, A., Fennessy, M. Carbon storage in US wetlands (2016) Nat Commun 7:13835. https://doi.org/10.1038/ncomms13835
Needelman BA, Emmer IM, Emmett-Mattox S, Crooks S, Megonigal JP, Myers D, Oreska MPJ, McGlathery K (2018) The science and policy of the verified carbon standard methodology for tidal wetland and seagrass restoration. Estuaries Coasts 41(8):2159–2171. https://doi.org/10.1007/s12237-018-0429-0
Neubauer SC, Givler K, Valentine S, Megonigal JP (2005) Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86(12):3334–3344. https://doi.org/10.1890/04-1951
Neubauer SC, Megonigal JP (2015) Moving beyond global warming potentials to quantify the climatic role of. Ecosystems. https://doi.org/10.1007/s10021-015-9879-4
Noyce GL, Megonigal JP (2021) Biogeochemical and plant trait mechanisms drive enhanced methane emissions in response to whole-ecosystem warming. Biogeosciences 18(8):2449–2463. https://doi.org/10.5194/bg-18-2449-2021
Perry JE, Hershner CH (1999) Temporal changes in the vegetation pattern in a tidal freshwater marsh. Wetlands 19(1):90–99. https://doi.org/10.1007/bf03161737
Pitz SA, Megonigal JP (2017) Temperate forest methane sink diminished by tree emissions. New Phytol 214(4):1432–1439. https://doi.org/10.1111/nph.14559
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31:831–842. https://doi.org/10.1007/s13157-011-0197-0
Purvaja R, Ramesh R (2001) Natural and anthropogenic methane emission from coastal wetlands of South India. Environ Manage 27(4):547–557
Rabenhorst MC, Megonigal JP, Keller JK (2010) Synthetic iron oxides for documenting sulfide in marsh pore water. Soil Sci Soc Am J 74(4):1383–1388. https://doi.org/10.2136/sssaj2009.0435
Roden E, Wetzel R (2003) Competition between Fe(III)-reducing and methanogenic bacteria for acetate in iron-rich freshwater sediments. Microb Ecol 45(3):252–258. https://doi.org/10.1007/s00248-002-1037-9
Saunois et al (2020) The global methane budget 2000–2017. Earth Syst Sci Data 12:1561–1623. https://doi.org/10.5194/essd-12-1561-2020
Solomon S, Qin D, Manning M et al (2007) IPCC fourth assessment report (AR4). Retrieved 4:2011
Sorrell BK, Brix H, DeLaune RD, et al (2013) Gas transport and exchange through Wetland Plant Aerenchyma. In: SSSA Book Series. Soil science society of America
Sorrell B, Mendelssohn IA, McKee KL, Woods RA (2000) Ecophysiology of Wetland plant roots: a modelling comparison of aeration in relation to species distribution. Ann Bot 86(3):675–685. https://doi.org/10.1006/anbo.2000.1173
Sutton-Grier AE, Megonigal JP (2011) Plant species traits regulate methane production in freshwater wetland soils. Soil Biol Biochem 43:413–420. https://doi.org/10.1016/j.soilbio.2010.11.009
Tuxen K, Schile L, Stralberg D et al (2011) Mapping changes in tidal wetland vegetation composition and pattern across a salinity gradient using high spatial resolution imagery. Wetlands Ecol Manage 19:141–157. https://doi.org/10.1007/s11273-010-9207-x
Vann CD, Megonigal JP (2003) Elevated CO2 and water depth regulation of methane emissions: Comparison of woody and non-woody wetland plant species. Biogeochemistry 63:117–134. https://doi.org/10.1023/A:1023397032331
Villa JA, Ju Y, Stephen T, Rey-Sanchez C, Wrighton KC, Bohrer G (2020) Plant-mediated methane transport in emergent and floating-leaved species of a temperate freshwater mineral-soil wetland. Limnol Oceanogr 65(7):1635–1650. https://doi.org/10.1002/lno.11467
Walker DA, Auerbach NA, Bockheim JG et al (1998) Energy and trace-gas fluxes across a soil pH boundary in the Arctic. Nature 394:469–472. https://doi.org/10.1038/28839
Wang Z, Zeng D Jr, WHP (1996) Methane emissions from natural wetlands. Environ Monit Assess 42:143–161. https://doi.org/10.1007/BF00394047
Weiss JV, Emerson D, Backer SM, Megonigal JP (2003) Enumeration of Fe(II)-oxidizing and Fe(III)-reducing bacteria in the root zone of wetland plants: implications for a rhizosphere iron cycle. Biogeochemistry 64:77–96
Weiss JV, Emerson D, Megonigal JP (2004) Geochemical control of microbial Fe(III) reduction potential in wetlands: Comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol Ecol 48(1):89–100. https://doi.org/10.1016/j.femsec.2003.12.014
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
Windham L (2001) Comparison of biomass production and decomposition between Phragmites australis (common reed) and Spartina patens (salt hay grass) in brackish tidal marshes of New Jersey, USA. Wetlands 21:179–188. https://doi.org/10.1672/0277-5212(2001)021[0179:COBPAD]2.0.CO;2
Yu K, Hiscox A, DeLaune RD et al (2013) Greenhouse gas emission by static chamber and eddy flux methods. In: DeLaune RD, Reddy KR, Richardson CJ, Megonigal JP (eds) Methods in biogeochemistry of wetlands, vol 10, SSSA Book Series. Soil Science Society of America, Madison, WI
Yuan J, Ding W, Liu D, Kang H, Freeman C, Xiang J, Lin Y (2015) Exotic Spartina alterniflora invasion alters ecosystem-atmosphere exchange of CH4 and N2O and carbon sequestration in a coastal salt marsh in China. Glob Chang Biol 21(4):1567–1580. https://doi.org/10.1111/gcb.12797
Acknowledgements
We thank the Chesapeake Bay MD National Estuarine Research Reserve Staff, and MD Department of Natural Resources for site access, support and accommodations for this research. We also thank Z. Bernstein, D. Leason, M. Molina, Z. Spencer, K. Willson, and M. Umanzor for their assistance in field data collection and sample processing. We thank the staff of the SERC Biogeochemistry lab for assistance with sample processing and preparation, G. Guntenspergen and USGS Staff for their assistance with porewater chemistry sampling equipment, and G. Seibel of UMD ENST for chamber collar construction.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Hatch project 1013805, the NSF Research Coordination Network Program of the Ecosystems Science Cluster (DEB-1655622), the Smithsonian Institution, and the Garden Club of America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Code availability (software application or custom code)
Not applicable.
Additional information
Responsible Editor: Adam Langley.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Derby, R.K., Needelman, B.A., Roden, A.A. et al. Vegetation and hydrology stratification as proxies to estimate methane emission from tidal marshes. Biogeochemistry 157, 227–243 (2022). https://doi.org/10.1007/s10533-021-00870-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-021-00870-z