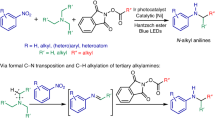
Abstract
Despite the widespread use of anilines, synthetic challenges to these targets still exist. Selectivity is often an issue, when using the traditional nitration-reduction sequence or more modern approaches, including arene C–H aminations catalyzed by transition metals, photosensitizers, or electrodes. Accordingly, there is still a need for general methods to rapidly, directly access specific isomers of substituted anilines. Here, we report a simple route towards the synthesis of such motifs starting from benzyl alcohols, which are converted to anilines by the use of arylsulfonyl hydroxylamines, via an aza-Hock rearrangement. Good to excellent yields are observed. The method is applicable to various benzyl alcohol surrogates (such as ethers, esters, and halides) as well as simple alkylarenes. Functionalizations of pharmaceutically relevant structures are feasible under the reaction conditions. Over ten amination reagents can be used, which facilitates the rapid assembly of a vast set of compounds.
Similar content being viewed by others
Introduction
Anilines are of paramount importance to all aspects of chemical science (pharmaceuticals, agrochemicals, organic chemicals, and natural bioactive products)1,2,3,4,5,6. Such motifs are typically accessed by a nitration-reduction sequence or by transition metal-catalyzed cross-coupling reactions (Buchwald-Hartwig7,8, Ullmann9,10,11, and Chan-Lam reaction12,13,14). In the case of cross-couplings, pre-functionalized aryl (pseudo) halides are required. To address this drawback, modern methods for direct arene C–H aminations via organometallic chemistry15,16, photochemistry17, and electrochemistry18 were invented. However, specific site-selectivity is a significant challenge for all of the mentioned protocols—ortho- versus para-selectivity for electron-rich substrates; ortho- versus meta- versus para-selectivity for electron-deficient substrates.
Alternatively, direct C–C amination is a method to address the site-selectivity problem. Many examples for transition metal-catalyzed functionalizations via decarboxylative aminations19 as well as metal-free reactions (Lossen20,21, Hoffmann22, Curtius23, Schmidt24 and Neber rearrangements25) from carboxylic acids and their derivatives exist, but several synthetic steps are needed. Only a few publications report on direct access to anilines under metal-free conditions in a one-step approach. The Beckmann rearrangement26,27 is a classical way to afford anilines from ketoximes or surrogates. A pioneering work reported by Walter28 relied on a similar strategy starting from 2-benzoylpyridine ketoxime or its O-p-toluenesulfonate, followed by a Beckmann rearrangement and hydrolysis to provide 2-amino pyridine or anilines, but only a limited number of examples were presented. A recent patent29 described anilines synthesis using the Beckmann rearrangement of aryl ketoximes, which still needed conc. HCl and high temperature (100 °C) to facilitate the transformation. Later this method was improved by Uchida30, who realized the direct deacylative amination of acyl arenes with ketoxime benzenesulfonate, affording anilines through a cascade reaction under mild conditions. However, via this methodology only primary anilines are accessible. Still, metal-free direct dealkylative aminations remain an underexplored area, especially for the difficult C(sp2)–C(sp3) bond cleavage (bond dissociation energy: C(sp2)–C(sp3) 98–100 kcal/mol)31,32. One other way to address this problem is the Schmidt rearrangement of α-azido ethylbenzene intermediates. An initial intramolecular C–C amination was enabled from benzylic azides in the presence of Lewis acids (CF3SO3H or EtAlCl2) as the catalyst, in which C–C aminated products served as side-products33. Later the Ning group34,35 showed that alkylarenes or secondary benzyl alcohols were feasible to undergo C–C aminations with DDQ as oxidant and NaN3 or alkyl azides as the aminating reagents. This is especially remarkable as this paves the way for these types of aminations starting from alcohols or alkylarene substrates instead of the commonly used ketones or carboxylic acids. Later, this technique was developed further by the same group36, replacing common oxidants with electrochemistry. However, highly toxic and explosive sodium azides or alkyl azides are essentially needed for this Schmidt-like transformation, thus safety precautions should be taken when undertaking such reactions. As a consequence, safe and easy-to-handle protocols are still needed for direct C–C aminations. Our approach was influenced by the synthesis of phenols via the Hock rearrangement, which is the key step for the industrial phenol synthesis (named Cumene-Phenol process or Hock process)37, the basis for the production of millions of tons of phenol every year (Fig. 1a). In the Hock rearrangement, cumene hydroperoxide, as a key intermediate, is transformed into phenol in acidic solvent after rearrangement and hydrolysis. A related migration of an aryl group in this case onto a nitrogen atom was realized by Falck and Kürti38, who used reactive ArB(OH)2NH2OX intermediates bearing a weak N–O single bond as a pathway to primary anilines. The key intermediates were generated by the nucleophilic attack of hydroxylamine derivatives onto arylboronic acids. Inspired by this technique, we hypothesized that cumene hydroxylamine derivatives, owing to the weak N–O bond (similar to the O-O bond in cumene hydroperoxide), are susceptible to a Hock-type rearrangement in an acidic solvent, yielding anilines as products (so-called aza-Hock rearrangement, Fig. 1b). Evidence that an aza-Hock rearrangement is feasible can be obtained by Hoffmann’s report39, showing that N-alkyl-O-(arylsulfonyl)hydroxylamines undergo cationic carbon-to-nitrogen rearrangements to form imines, which upon hydrolysis generate anilines (two examples). Here we present a mild, general and scalable, chemoselective method for C–C aminations. It has a broad scope, including secondary benzyl alcohols, simple alkylarenes in combination with arylsulfonyl hydroxylamines (ArSO2ONHR) as aminating reagents, delivering primary or secondary anilines in good to excellent yields (>70 examples, Fig. 1c).
Color labels are used to visualize the disconnection. a Hock rearrangement for phenol synthesis. b Our proposal for aniline synthesis. c This work with direct C–C amination via aza-Hock rearrangement. DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, HFIP hexafluoroisopropanol, TFE trifluoroethanol, TFA trifluoroacetic acid.
Results
Reaction design and optimization towards secondary anilines
Hydroxylamines and their derivatives are powerful aminating reagents, which are often used for arene C–H and X-H aminations (X = O, N, S, P)40,41,42,43,44,45 as well as Schmidt-type reaction46, serving as alternative ways to introduce amino groups on various chemical skeletons. Recently, our group47 published a metal-free arene C–H amination using ArSO2ONHR in HFIP. All this prompted us to investigate the possibility of harnessing hydroxylamine derivatives for direct arene dealkylative aminations. We supposed that 2-phenylethanol, as an electrophilic precursor in fluorinated alcoholic solvents, could react with nucleophilic TsONHMe to form the reactive Hock-type intermediate, which triggered by the weak N–O bond in situ might undergo an aza-Hock rearrangement, which after hydrolysis of an intermediate iminium ion would give valuable anilines. Already the initial exploratory experiment using 1-(4-methoxyphenyl)ethan-1-ol 1a and TsONHMe 2a led to aniline 3a in a good yield (Table 1, entry 6). The aminating reagent 2a (0.22 mmol) in combination with 0.2 mol/L 1a in HFIP turned out to be the optimal reaction condition and 74% isolated yield was obtained after the systematic screening. The choice of solvent was crucial: nonfluorinated polar or nonpolar solvents were not effective, but fluorinated alcohols worked well and HFIP proved to be superior to TFE (entries 1–5). A larger excess of the aminating reagent and higher concentrations slightly increased the yield (entries 5–7 and entries 6, 8, 9). A nitrogen atmosphere or exclusion of light did not affect the C–C amination (entry 10, 11) and the addition of p-toluenesulfonic acid or TFA suppressed the formation of anilines due to the competing water elimination from the alcohol (entry 12, 13).
Substrate scope in the synthesis of secondary anilines
The scope of this dealkylative amination is striking and almost all tested secondary benzyl alcohol partners were transformed in excellent chemoselectivity. Alcohols that can be used in the C–N bond formation are exemplified in Fig. 2. Commercially available secondary benzyl alcohols (1a–1d) proceeded smoothly with the protocol. Higher yields were obtained when electron-donating groups were installed on the arene core. Tertiary alcohols (1e, 1f) also reacted with 2a, providing excellent yields. Notably, the electron-rich 4-(methylamino)phenol 3g, which is sensitive to oxidants, was successfully synthesized with the aid of the related aminating reagent MsONHMe 2b, which was first used in C–H amination by our group47. Alcohols (1h–1j) bearing electron-withdrawing groups (Br, Cl, OTs) were also compatible. In the case of strong electron-withdrawing groups (CN, 1k; NO2, 1l), an additional methoxyl group on the benzene core was essential to regulate the electronic properties. 1-(3-(Methylamino)phenyl)ethan-1-ol 3m was the only product when bifunctional 1,1’-(1,3-phenylene)bis(ethan-1-ol) 1m was implemented in the reaction, which we attribute to the iminium salt for aniline 3m in HFIP (See discussion). Di- or tri-substituted alcohols (1n, 1o) were also tested and the formation of C–N bonds was achieved in good yields, especially hindered alcohol 1o was also tolerated. Biphenyl- and diphenyl ether substructures were well tolerated as well as naphthalene (1p–1r). It needs to be pointed out that 4-bromo-N-methylbenzene 3s in combination with 4-bromobenzaldehyde in 70% isolated yield (key side-product for the reaction, see mechanistic discussion below) was accessed when diphenyl methanol 1s was treated with 2a. Moreover, heteroarenes and heterocycles (1t–1x) were viable for the transformation, even the triple bond of 3v was kept intact in the presence of oxidative 2a. Noteworthy, in contrast to diphenyl ether 1q, the fused benzene ring 1t delivered a better yield, which is attributed to the participation of lone pair in the aromaticity of the five-member ring.
Given these collective findings, we anticipated that our protocol would be amenable for late-stage functionalizations of natural products, drugs, or chemical waste. Fragrances (tonalide 1 y, coumarin 1z) was therefore prepared and exposed to the aminating reagent 2a, providing the products in moderate to good yield; however, for coumarin, a higher concentration of 2a was needed to achieve the dealkylative amination and inhibit the formation of coumarin ether as byproduct (see SI). Drugs (gemfibrozil 1aa, naproxen 1ab, and fenofibric acid 1ac) were also tested under the standard conditions, delivering the amination products in good yield; and again, the aldehyde was also isolated from the reaction of 1ac and 2a (see SI). Bio-renewable lignin 1ad also underwent the C–C amination in a good yield, which opens up a window for the synthesis of valuable anilines from waste chemicals. The aminating reagent 2a could also selectively cleave the C–C bond of benzyl alcohols attached to natural products (estrone, 1ae), leaving the aliphatic alcohol intact. The robust and practical nature of our strategy was also demonstrated by the large-scale preparation of 3 h in almost the same efficiency. Furthermore, olefin and other alcohols were also investigated for the C–C amination: α-alkyl substituted styrene, 1-(1-cyclopropylvinyl)-4-methoxybenzene 1af, was smoothly transformed into aniline 3a in moderate yield; 1-(4-methoxyphenyl)propan-2-ol 1ag gave the C–H amination rather than C–C amination product when treated with 2a, while (4-methoxyphenyl)methanol as primary benzyl alcohol did not react with 2a.
As a next step, we evaluated the possibility of introducing different N-substituents via the hydroxylamine derivatives. 12 different aminating reagents (2b or ArSO2ONHR, the latter accessed via Mitsunobu reactions) were evaluated for this method. As depicted in Fig. 3, various secondary anilines (3a, 4c–4i) were synthesized from hydroxylamines (2b–2i) in excellent yields, even for sensitive aminating reagents (2c, 2d) and sterically hindered reagents (2e, 2f). An alkyl chloride was kept intact during the synthesis of 4i. A carbamate group 4j was successfully installed on the benzene core when 1a was treated with 2j in less acidic TFE, and a propargyl amine 4k unit was smoothly introduced to anisole. It is noteworthy that the intermolecular C–C aminations delivered products 4l, 4m in excellent yield, no intramolecular aziridination48 or arene C–H amination49 side-products were observed despite the reported aziridination of 2l and the arene C–H amination of 2m in HFIP or TFE. A tertiary aniline derivative was not accessible with TsONMe2 as aminating reagent under these conditions.
Reaction optimization towards primary anilines
Next, we wanted to address the even more challenging synthesis of primary anilines via the C–C amination protocol (Fig. 4). First, over 20 aminating reagents were tested. The optimization of the direct conversion to primary aniline from benzyl alcohol is summarized in Table 2. N-Boc arylsulfonyl hydroxylamines (5a–5g) or MsONHBoc 5s delivered the dealkylative amination products in moderate yields (entries 1–7, 19); among all of the tested ArSO2ONHBoc reagents, the sterically hindered tert-butyl (((2,4,6-triisopropylphenyl)sulfonyl)oxy)carbamate 5e demonstrated the best performance, affording a moderate yield (50%) in HFIP. However, O-benzoyl or pivaloyl hydroxylamines (5h–5j, 5r) only led to minor or trace amounts of product (entries 8–10, 18). Besides, other commercially available reagents like (2,4-dinitrophenyl)hydroxylamine (DNPHA, 5k) and O-(diphenylphosphinyl)hydroxylamine 5l with a free amino group were less effective for the amination (entry 11, 12). Unprotected O-sulfonyl hydroxylamines turned out to be comparably effective than their corresponding N-Boc derivatives (entry 4 and 13; entry 5 and 14; entry 6 and 15). Noteworthy, O-mesitylenesulfonylhydroxylamine (MSH, 5m) appeared to be the optimal reagent for this conversion in a 59% yield (entry 13, MSH could be stored at −20°C for 1 month with only a little decomposition). Less acidic TFE was confirmed to be a better solvent than HFIP (5% higher yield), which can be attributed to the reduced formation of the elimination product from the alcohol which turned out to be a side pathway (entry 13, 22). Overall, 0.3 mmol of 5m with 0.2 mol/L alcohol in TFE turned out to be the best conditions for the conversion (79% isolated yield, entry 24). Furthermore, the frequently applied commercially available hydroxylamine-O-sulfonic acid (5q) delivered only a low yield and TMSONH2 5p was not effective (entry 16, 17). Hydroxylamine triflate salts (5t, 5u) only achieved moderate yields, which we attribute to the formation of free triflic acid which can decompose the starting material (entry 20, 21).
Substrate scope in the synthesis of primary anilines
Under the optimized conditions, we next investigated the scope of this methodology (Fig. 5). In a series of benzyl alcohols, with an increasing electron density at the aromatic core, primary anilines were obtained from low to good yields (6a, 6d, 6ai). Substrates with electron-withdrawing groups (F, 1aj; NHAc, 1ak) could be converted with HFIP as the solvent. Di- or tri-substituted alcohols (1n, 1al, 1o) were amenable for the dealkylative amination in good yields, including sterically hindered substrate 1o. Besides, biphenyl, naphthalene, and diphenyl ether (1p–1r) were tolerated. Not surprisingly, diphenyl methanol 1s was cleaved by 5m, forming the desired aniline 6s. Heterocycles (1t–1x) were also competent transformation partners under the reaction conditions as well as pyridine 1am and thiophene 1an. The applicability of the methodology for late-stage modifications of drug leads or natural products was demonstrated next. Secondary alcohols derived from fragrances (veratraldehyde 1ah and tonalide 1y), drugs (gemfibrozil 1aa, naproxen 1ab, and fenofibric acid 1ac) and a natural product (estrone 1ae) were successfully converted to anilines, giving good yields. The scalability of the reaction was made evident by the preparation of aniline 6a on a 4 mmol scale.
Synthetic applications
Further applications using our protocols are illustrated in Fig. 6. Our methodology furnished 2-phenyl anilines (7, 8) by a direct C–C amination in just one step. These targets are used as commodity Buchwald ligands precursors (for G2–G4 type ligands). The formal total synthesis of Lidocaine50 (from 9) and Chlorambucil51 (from 10) could be acquired in very short sequences. Ether and esters (11, 12) (as alternative precursors for benzylic cations) were also useful substrates for the anilines synthesis. Tertiary aniline 13 was affordable by a sequence of a C–C amination of 2a, followed by a subsequent reduction with NaBH3CN in one pot. This not only strongly supports the formation of an intermediate imine in the reaction but also further contributes to the synthetic potential of the methodology. Furthermore, under our conditions C–C oxygenations and C–C brominations were also possible. A phenol 14 synthesis from alcohol was feasible when hydrogen peroxide was used instead of a hydroxylamine derivative and a bromobenzene 15 was obtained if NBS under irradiation with UV light was applied.
a Four aniline examples for catalysis/bioactive molecules precursors. b Benzyl ether/ester as substrate in the aza-Hock rearrangement. c Tertiary aniline synthesis with this protocol. d Phenol/aryl bromide synthesis with a similar strategy. Reaction conditions: alcohol (0.2 mmol), 2a (0.22 mmol) or 5 m (0.3 mmol), HFIP (hexafluoroisopropanol) (1 mL) or TFE (trifluoroethanol) (1 mL), 12 h, r.t.; Ts toluenesulfonyl, NBS N-bromosuccinimide.
Mechanistic investigation
Concerning the mechanism, we initially considered that a radical pathway might operate related to our previous work47. However, all attempts to trap or detect radicals failed (see SI). In order to get more proof, further control experiments were conducted (Fig. 7). Aniline 3h was also accessible via the bromide derivative of 2-phenylethanol 16 by our protocol. This gives a strong indication that a benzyl cation is the key intermediate of the reaction (the formation of such a cation is a well-known process for benzyl alcohol and halide in HFIP52,53,54,55). More support for the formation of a cation was provided by the formation of ether 17 from the electron-deficient alcohol 1i. Furthermore, a direct dealkylative amination was achieved from 4-isopropyl anisole 18 following a pathway related to Ning’s strategy35. Besides, 4-bromobenzaldehyde was obtained as a byproduct when diphenyl methanol was treated with 2a, which proves strong support for the imine hydrolysis. Moreover, a Beckmann rearrangement could be excluded for this reaction, as no aniline was detected when ketone 20 was applied as a substrate under the standard conditions. Accordingly, we propose that the reaction proceeds via an aza-Hock rearrangement in four steps: generation of a benzyl cation via benzyl alcohol solvolysis by HFIP; formation of a reactive O-(1-phenylethyl)hydroxylamine, which gives access to an iminium tosylate salt after aryl migration; and finally the irreversible hydrolysis of the imine yielding the desired aniline after simple workup (Fig. 8). The hydrolysis of protonated imines in the reaction mixture only in the workup with sodium hydrogen carbonate (not in situ with the one equivalent of neutral water formed in the reaction–compare also the selective formation of 13 in Fig. 6, which is based on the same effect), gives an explanation for the selective cleave of one C–C bond instead of two C–C bonds in substrates with two reactive benzylic alcohol groups (1m selectively forms 3m); the iminium group in the intermediate iminium tosylate electronically de-activates the arene ring, the second benzylic alcohol does not react anymore.
Discussion
In conclusion, the mild conditions, operational simplicity, and perfect chemoselectivity are the attributes of the C–C amination of benzylic alcohols with ArSO2ONHR to anilines, which in our opinion is not only attractive for academia but also for industry. As already demonstrated, other precursors for benzylic cations also serve as precursors, which further expands the synthetic utility. In addition, chemoselective C–C brominations and oxygenations are possible under the same conditions. Mechanistically, an aza-Hock rearrangement is proposed by us. Interestingly, despite some early evidence for such a reactivity pattern, until known the synthetic utility of this process was limited and our report might pave the way for further protocols based on this pattern in the future.
Methods
General procedure for the synthesis of secondary anilines
To a solution of the alcohol (0.2 mmol) in 1.0 mL HFIP was added aminating reagent (0.22 mmol) at room temperature under ambient atmosphere unless otherwise stated. The reaction was stirred at room temperature for 12 h (monitored by GCMS or TLC). The reaction was diluted with 1 mL DCM and basified with 1 mL saturated NaHCO3 aqueous solution. The aqueous layer was extracted with DCM (3 mL × 3), and the combined organic layers were washed with 5 mL sat. brine, dried over anhydrous Na2SO4, filtrated, and concentrated in vacuo. The crude residue was purified by silica gel chromatography with PE/EA (petroleum ether/ethyl acetate) to afford the desired product.
General procedure for the synthesis of primary anilines
To a solution of the alcohol (0.2 mmol) in 1.0 mL TFE was added MSH (0.3 mmol) under ambient atmosphere at room temperature unless otherwise stated. The reaction was stirred at room temperature for 12 h (monitored by GCMS or TLC). Then the reaction was diluted with 1 mL DCM and basified with 1 mL saturated NaHCO3 aqueous solution. The aqueous layer was extracted with DCM (3 mL × 3), and the combined organic layers were washed with 5 mL sat. brine, dried over anhydrous Na2SO4, filtrated, and concentrated in vacuo. The crude residue was purified by silica gel chromatography with PE/EA to afford the desired product.
Data availability
Authors can confirm that all relevant data are included in the paper and/ or its supplementary information files.
References
Scholz, U. & Schlummer, B. Arylamines. In Science of Synthesis: Houben–Weyl methods of molecular transformations Vol. 31b 1565–1678 (Georg Thieme Verlag, 2007).
Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences. (Wiley-VCH, 2008).
McGrath, N. A., Brichacek, M. & Njardarson, J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Cernak, T. et al. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Hartwig, J. F. Carbon−heteroatom bond-forming reductive eliminations of amines, ethers, and sulfides. Acc. Chem. Res. 31, 852–860 (1998).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C-N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Ullmann, F. Ueber eine neue bildungsweise von diphenylaminderivaten. Ber. Dtsch. Chem. Ges. 36, 2382–2384 (1903).
Goldberg, I. Ueber phenylirungen bei gegenwart von kupfer als katalysator. Ber. Dtsch. Chem. Ges. 39, 1691–1692 (1906).
Sambiagio, C., Marsden, S. P., Blacker, A. J. & McGowan, P. C. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 43, 3525–3550 (2014).
Chan, D. M. T., Monaco, K. L., Wang, R.-P. & Winters, M. P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 39, 2933–2936 (1998).
Evans, D. A., Katz, J. L. & West, T. R. Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic acids. An expedient synthesis of thyroxine. Tetrahedron Lett. 39, 2937–2940 (1998).
Lam, P. Y. S. et al. New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 39, 2941–2944 (1998).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Jiao, J., Murakami, K. & Itami, K. Catalytic methods for aromatic C–H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 6, 610–633 (2016).
Zhao, Y. & Xia, W. Recent advances in radical-based C-N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 47, 2591–2608 (2018).
Karkas, M. D. Electrochemical strategies for C–H functionalization and C-N bond formation. Chem. Soc. Rev. 47, 5786–5865 (2018).
Arshadi, S. et al. Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N–H compounds. RSC Adv. 9, 8964–8976 (2019).
Lossen, W. Ueber benzoylderivate des hydroxylamins. Ann. Chem. Pharm. 161, 347–362 (1872).
Yale, H. L. The hydroxamic acids. Chem. Rev. 33, 209–256 (1943).
Hofmann, A. W. Ueber die einwirkung des broms in alkalischer lösung auf amide. Ber. Dtsch. Chem. Ges. 14, 2725–2736 (1881).
Curtius, T. Ueber stickstoffwasserstoffsäure (azoimid) N3H. Ber. Dtsch. Chem. Ges. 23, 3023–3033 (1890).
Wolff, H. The Schmidt reaction. in Organic Reactions 307–336 (2011).
Wallace, R. G., Barker, J. M. & Wood, M. L. Migration to electron-deficient nitrogen. a one pot synthesis of aromatic and heteroaromatic amines from carboxylic acids. Synthesis 1990, 1143–1144 (1990).
Beckmann, E. Zur kenntniss der isonitrosoverbindungen. Ber. Dtsch. Chem. Ges. 19, 988–993 (1886).
Gawley, R. E. The Beckmann reactions: rearrangements, elimination-additions, fragmentations, and rearrangement-cyclizations. In Organic Reactions, 1–75 (1988).
Huntress, E. H. & Walter, H. C. Beckmann rearrangement of the oximes of phenyl 2-pyridyl ketone (2-benzoylpyridine). J. Am. Chem. Soc. 70, 3702–3707 (1948).
Chen, F. One-pot method for preparing aromatic amine using aromatic ketones and proton acid catalyst. CN 108047059A (2018).
Hyodo, K., Hasegawa, G., Maki, H. & Uchida, K. Deacetylative amination of acetyl arenes and alkanes with C–C bond cleavage. Org. Lett. 21, 2818–2822 (2019).
McMillen, D. F. & Golden, D. M. Hydrocarbon bond dissociation energies. Annu. Rev. Phys. Chem. 33, 493–532 (1982).
Brocks, J. J., Beckhaus, H.-D., Beckwith, A. L. J. & Rüchardt, C. Estimation of bond dissociation energies and radical stabilization energies by ESR spectroscopy. J. Org. Chem. 63, 1935–1943 (1998).
Murali, A., Puppala, M., Varghese, B. & Baskaran, S. A lewis acid mediated schmidt reaction of benzylic azide: synthesis of sterically crowded aromatic tertiary amines. Eur. J. Org. Chem. 2011, 5297–5302 (2011).
Qin, C., Shen, T., Tang, C. & Jiao, N. FeCl2-promoted cleavage of the unactivated C–C bond of alkylarenes and polystyrene: direct synthesis of arylamines. Angew. Chem. Int. Ed. 51, 6971–6975 (2012).
Liu, J. et al. From alkylarenes to anilines via site-directed carbon-carbon amination. Nat. Chem. 11, 71–77 (2019).
Adeli, Y. et al. Electrochemically oxidative C–C bond cleavage of alkylarenes for anilines synthesis. ACS Catal. 9, 2063–2067 (2019).
Hock, H. & Lang, S. Autoxydation von kohlenwasserstoffen, IX. mitteil.: über peroxyde von benzol-derivaten. Ber. Dtsch. Chem. Ges. 77, 257–264 (1944).
Zhu, C. et al. Elusive metal-free primary amination of arylboronic acids: synthetic studies and mechanism by density functional theory. J. Am. Chem. Soc. 134, 18253–18256 (2012).
Hoffman, R. V. & Kumar, A. Cationic carbon-to-nitrogen rearrangements in N-(arylsulfonoxy)amines. J. Org. Chem. 50, 1859–1863 (2002).
Tamura, Y., Minamikawa, J. & Ikeda, M. O-Mesitylenesulfonylhydroxylamine and related compounds - powerful aminating reagents. Synthesis 1977, 1–17 (1977).
Sun, R. et al. Design, synthesis, bioactivity, and structure-activity relationship (SAR) studies of novel benzoylphenylureas containing oxime ether group. J. Agric. Food Chem. 56, 11376–11391 (2008).
Sabir, S., Kumar, G. & Jat, J. L. O-Substituted hydroxyl amine reagents: an overview of recent synthetic advances. Org. Biomol. Chem. 16, 3314–3327 (2018).
Legnani, L., Prina Cerai, G. & Morandi, B. Direct and practical synthesis of primary anilines through iron-catalyzed C–H bond amination. ACS Catal. 6, 8162–8165 (2016).
Liu, J. et al. Fe-catalyzed amination of (hetero)arenes with a redox-active aminating reagent under mild conditions. Chem. Eur. J. 23, 563–567 (2017).
D’Amato, E. M., Borgel, J. & Ritter, T. Aromatic C–H amination in hexafluoroisopropanol. Chem. Sci. 10, 2424–2428 (2019).
Liu, J. et al. Nitromethane as a nitrogen donor in Schmidt-type formation of amides and nitriles. Science 367, 281–285 (2020).
Wang, T. et al. A metal‐free direct arene C−H amination. Adv. Synth. Catal. 363, 2783–2795 (2021).
Farndon, J. J., Young, T. A. & Bower, J. F. Stereospecific alkene aziridination using a bifunctional amino-reagent: an aza-prilezhaev reaction. J. Am. Chem. Soc. 140, 17846–17850 (2018).
Farndon, J. J., Ma, X. & Bower, J. F. Transition metal free C-N bond forming dearomatizations and aryl C–H aminations by in situ release of a hydroxylamine-based aminating agent. J. Am. Chem. Soc. 139, 14005–14008 (2017).
S, U. D. et al. A photochemical dehydrogenative strategy for aniline synthesis. Nature 584, 75–81 (2020).
Gao, X. et al. Novel enmein-type diterpenoid hybrids coupled with nitrogen mustards: synthesis of promising candidates for anticancer therapeutics. Eur. J. Med. Chem. 146, 588–598 (2018).
Colomer, I., Chamberlain, A. E. R., Haughey, M. B. & Donohoe, T. J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 1, 0088 (2017).
An, X. D. & Xiao, J. Fluorinated alcohols: magic reaction medium and promoters for organic synthesis. Chem. Rec. 20, 142–161 (2020).
Trillo, P., Baeza, A. & Najera, C. Fluorinated alcohols as promoters for the metal-free direct substitution reaction of allylic alcohols with nitrogenated, silylated, and carbon nucleophiles. J. Org. Chem. 77, 7344–7354 (2012).
Zhang, F. Z., Tian, Y., Li, G. X. & Qu, J. Intramolecular etherification and polyene cyclization of pi-activated alcohols promoted by hot water. J. Org. Chem. 80, 1107–1115 (2015).
Acknowledgements
T.W., H.S. and C.H. are grateful for Ph.D. fellowships from the China Scholarship Council (CSC). We also thank Maximilian Elter for the technical support; P.M.S. and A.S.K.H. gratefully acknowledge the Hector Fellow Academy for the generous provision of funding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T.W. designed, carried out the main part of the experiments, and analyzed data; P.M.S. contributed several experiments to the mechanistic part; All authors participated in discussion; T.W., M.R., and Dr. A.S.K. Hashmi co-wrote the paper; M.R. and A.S.K Hashmi supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, T., Stein, P.M., Shi, H. et al. Hydroxylamine-mediated C–C amination via an aza-hock rearrangement. Nat Commun 12, 7029 (2021). https://doi.org/10.1038/s41467-021-27271-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-27271-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.