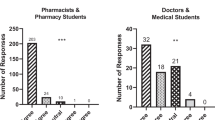
Abstract
Background
The adoption of oncology biosimilars has been slow in the USA, which may be attributed in part to stakeholder perceptions and lack of operational guidance that supports favorable access to biosimilars.
Objective
Our objective was to understand the real-world implementation experiences with oncology biosimilars of US payers and healthcare professionals (HCPs) as their experience with biosimilars has evolved.
Methods
In-depth qualitative interviews with payers (n = 20) and HCPs (n = 17 physicians, n = 3 practice managers) were conducted. Payers included managed care organizations (MCOs), integrated delivery networks, and pharmacy benefit managers (PBMs). Physicians were affiliated with a healthcare network or were community based, specialized in hematology/oncology, and had prescribed oncology biosimilars. Audio transcripts of the interviews were coded using MaxQDA software to enable descriptive analysis of the qualitative data.
Results
Over 80.0% of physicians perceived the efficacy and safety of biosimilars to be highly comparable to that of originators. Up to 87.5% of physicians reported using biosimilars in > 50% of their treatment-naïve patients and were comfortable using biosimilars in all approved indications. To encourage utilization, 75.0% of MCOs/PBMs preferred biosimilars over originators in treatment-naïve patients and implementation via step therapy. Physician involvement in choosing biosimilars was minimal, which was largely dependent on practice protocols or insurance preferences. The major factor influencing payers’ coverage decisions and biosimilar adoption was potential cost savings.
Conclusions
US payers and physicians who have experience with biosimilars have favorable views of oncology biosimilars, particularly for treatment-naïve patients. A framework for integrating biosimilars into oncology practice is developing, primarily driven by insurance coverage, contracting, and cost benefits.
Similar content being viewed by others
Most physicians perceived the efficacy and safety of biosimilars to be highly comparable to that of originators and were comfortable using biosimilars in all approved indications. |
Most physicians reported using biosimilars with their treatment-naïve patients, and most payers encouraged utilization of biosimilars through step therapy. |
Potential cost saving was the major factor that influenced payer coverage decisions and physician biosimilar adoption. |
1 Introduction
Advances in targeted therapies have improved survival in many cancers but have also escalated the costs more rapidly. In 2019, biologics accounted for 43% of total medicine spending in the USA, and oncology biologics was the highest spending drug class [1]. A biosimilar is defined as a biologic product that is “highly similar to the reference product notwithstanding minor differences in clinically inactive components,” and one in which “there are no clinically meaningful differences between the biologic product and the reference product in terms of the safety, purity, and potency of the product” [2]. Biosimilars hold the promise of reducing healthcare costs and improving patient access to high-priced biologics. According to a RAND report, from 2017 to 2026, biosimilars were estimated to save $US54 billion (up to $US150 billion) on biologic drug spending, which equals approximately 3% of total estimated biologic spending in the USA [3]. From US payers’ perspective, a recent budget impact analysis showed that the introduction of the bevacizumab-bvzr biosimilar would lead to cost savings of $US7 million and $US4 million for a commercial payer and Medicare, respectively, over 5 years [4]. As of July 2021, ten of the 30 US FDA-approved biosimilars are oncology monoclonal antibodies (mAbs), including trastuzumab, bevacizumab, and rituximab [5].
However, unlike in Europe, the adoption of oncology biosimilars has been slow in the USA [6], which might be attributed to several factors unique to the US market, such as regulatory, legislative, and clinical frameworks, key stakeholder perceptions, and reimbursement arrangements [7]. Previous studies have pointed out that concerns surrounding biosimilar safety, efficacy, and extrapolation (i.e., when a biosimilar is approved for use in a reference product indication when that indication was not included in the biosimilar clinical trial) among physicians are some of the major barriers to biosimilar utilization [8,9,10,11]. According to a systematic review of studies published between 2014 and 2019 that evaluated physicians’ perceptions of biosimilars, 54–94% were confident prescribing biosimilars; however, 65–67% of physicians had concerns regarding these medicines [12]. Notably, only three of the 23 studies included were conducted in the USA, and all of these were conducted prior to the first oncology mAb biosimilar being approved by the FDA in 2017 [9, 13, 14], so results might not represent the current treatment dynamics in oncology care. Two other US-based surveys among community oncologists and academic oncologists [15, 16], respectively, showed a lack of understanding of biosimilars and the need for education, but both studies were conducted when oncology mAb biosimilars were new to the US market. Additionally, a more recent US-based survey study conducted from December 2019 to January 2020 among physicians in six different medical specialties, including oncology, likewise observed a limited understanding of biosimilarity, even among respondents who had previously prescribed a biosimilar [17]. However, most currently approved oncology mAb biosimilars had been approved for ≤ 6 months or had not yet been approved at the time that study was conducted. As physicians’ experience with oncology mAb biosimilars is evolving, it is important to assess current perceptions, knowledge, and prescribing in real-world practice among treating oncologists who already prescribe biosimilars, as this might provide operational guidance and therefore optimize the integration of oncology mAb biosimilars into routine clinical practice.
Another important factor affecting oncology biosimilar uptake in the USA is payers’ reimbursement policies and the formulary status for biosimilars [17,18,19], which further underscore the need to identify innovative approaches to maximize biosimilar uptake. To the best of our knowledge, no study has examined payers’ methods to control spending by promoting oncology mAb biosimilar usage in real-world practice as their experience has evolved with increased exposure to biosimilars.
Monitoring trends in the perceptions and knowledge of, and operational and prescribing experiences with, biosimilars over time is important in improving the adoption of oncology biosimilars across both treating oncologists and payers. Hence, the present study aimed to understand (1) perceptions and knowledge of three oncology mAbs: trastuzumab, bevacizumab, and rituximab biosimilars; (2) factors that affect selection of biosimilars versus their reference products; (3) methods employed to increase biosimilar adoption; and (4) operational considerations and experiences that are useful in guiding favorable access to biosimilars in the US oncology practice setting from the perspectives of both payers and treating oncologists.
2 Methods
2.1 Survey Participants
Physicians and practice managers were recruited by a large international agency that specializes in recruiting many audiences, including physicians. Physicians and practice managers must opt-in to participate in the panel. Panel members are recruited through multiple methods, and panel maintenance and verification are updated annually. Specifically, physicians were recruited by Schlesinger Associates, and practice managers were recruited by Guide Point, which also recruited integrated delivery network (IDN) payers and one participating clinical pharmacist from a managed care organization (MCO). Pharmacy benefit managers (PBMs) and all other participating MCO payers were recruited by an independent recruiter using a payer panel co-developed by Kantar with Ellis Consulting, an independent recruiter that has grown and maintained the panel for over 20 years. This co-developed payer panel focuses on MCO and PBM payers. All potential participants in this study were initially screened to confirm eligibility and had to provide informed consent to participate. The study protocol was determined to be exempt from expedited or full ethical review by Pearl Institutional Review Board (protocol: 20-KANT-236).
2.2 Qualitative Interviews
In-depth qualitative interviews lasting 45 min were conducted individually via telephone with 20 healthcare professionals (HCPs) (n = 17 treating oncologists/hematologists, n = 3 oncology practice managers) and 20 payers (n = 10 MCOs, n = 8 IDNs, n = 2 PBMs). Interviews with HCPs and practice managers were conducted between 12 October and 18 December 2020, and payer interviews were conducted between 5 and 26 October 2020. Practice manager interviews focused on questions related to the operational features of biosimilar adoption and use and excluded most questions related to clinical assessments. Participating HCPs and payers were paid honoraria for their time. All interviews were conducted by trained, experienced moderators and were audiotaped with the prior consent of participants. The use of qualitative research methodology means that results might not be generalizable to the entire targeted population; however, responses should represent US oncologists with some biosimilar experience.
2.3 Eligibility Criteria
2.3.1 Healthcare Professionals
To participate, physicians must have been a board-certified oncologist (medical or hematology) with ≥ 3 years in practice, and they must have spent ≥ 50% of their professional time dedicated to direct patient care. They must have been practicing in the USA in either an independent community setting with an onsite infusion suite, an outpatient academic setting, or an oncology practice in an IDN setting that treats patients with intravenous treatments. Physicians were included in the study if they had prescribed any trastuzumab, rituximab, and/or bevacizumab biosimilar to three or more patients in the 6 months before recruitment. Among the total physician sample, 76% (13/17), 65% (11/17), and 53% (9/17) had experience prescribing multiple trastuzumab, rituximab, and bevacizumab biosimilars available in the US market, respectively, and all the physicians (17/17 [100%]) had experience prescribing the trastuzumab, rituximab, and bevacizumab reference drugs.
Practice managers must have had high familiarity with key issues related to oncology infusions, especially in the context of biosimilar use, as well as with the financial implications of biosimilar use and protocols for biosimilars. They must have been a practice manager in a group oncology or oncology/hematology practice with onsite infusion suite capabilities; additionally, the practice in which they worked must have been an independent private community practice and not part of a health system or hospital system.
2.3.2 Payers
All payers must have been a member of a pharmacy and therapeutics (P&T) committee and/or medical therapeutic committee. They must have been fairly to highly knowledgeable about their parent organization’s decision-making process on the coverage of oncology biosimilars. Additionally, all payers must have had an existing reimbursement policy for some oncology biosimilars (if MCO) or included in the formulary for outpatient infusions (if IDN) for one or more of the biosimilars available for trastuzumab, rituximab, or bevacizumab reference products.
MCO and PBM payers must have been involved in formulary coverage and utilization management of a medical benefit plan either alone or in addition to pharmacy benefits for products used in oncology. Payers must cover ≥ 5 million lives, ≥ 350,000 lives, or ≥ 10 million lives if a national, regional, or PBM payer, respectively. One clinical pharmacist involved in the review of dossiers and other data for presentation at P&T committee was included in the study as an MCO. MCO and PBM payers were ineligible to participate if they were part of a hospital, hospital system, or retail pharmacy. IDN payers must have had 3–30 years of professional experience in their current role. Additionally, IDNs must have had a common outpatient formulary/policy across the system for biosimilar usage and must have been involved in processes regarding policy and utilization of biosimilars within the outpatient infusion centers.
2.4 Analysis
Verbatim transcripts were generated from the audio recordings for each participant and were reviewed to ensure that any personally identifying information was excluded and that transcripts accurately represented the audio recordings. MaxQDA software was used for coding the transcripts to enable the identification of key themes that emerged from the interviews. Specifically, the coder analyzed the transcript text and assigned the numeric code to each theme raised. The coder developed the initial sets of codes based on the first two interview transcripts for each respondent group. These codes were subsequently reviewed by the moderator to confirm the credibility and consistency of the emerging themes. The coded themes were input into the MaxQDA software for each transcript. Quotes related to each theme/concept were also added to the software, so that the actual language used is represented and associated with each coded response. The software consolidates the counts associated with each theme/concept, which allows development of the saturation grid for each respondent group based on the final datasets and then verified to ensure the interviews captured all possible themes. The saturation grid identifies how many respondents were interviewed before no new themes or concepts are identified. Typically, in qualitative research, saturation of new themes/concepts is found by the 12th respondent [20]. Each saturation grid consisted of a chart that facilitated the organization of the coded qualitative data and evaluation of these data for completeness by listing the study research questions against the themes generated from the interview data pertinent to each research question. All count variables, such as number of patients seen per month, were reported as means and standard deviations (SDs), with categorical data, including coded qualitative responses, reported as frequencies and percentages.
3 Results
3.1 Respondent Characteristics
Oncologists had a mean ± SD 19 ± 6 years in practice (Table 1). A majority were affiliated with either an outpatient academic/university hospital or a private/group practice (for each, 5/17 [29.4%]). On average, physicians saw 540 ± 227 patients per month at an infusion center. Practice managers reported overseeing practices with intakes of 65–1350 patients monthly at an infusion center and supervising 3–20 physicians. A majority of MCO/PBM (7/12 [58.3%]) and IDN (7/8 [87.5%]) payers were currently pharmacy directors/vice presidents and had 17 ± 6 and 16 ± 8 years of experience, respectively (Table 1). Most MCO/PBM payers (11/12 [91.7%]) managed both medical and pharmacy benefits.
3.2 Biosimilar Perceptions and Preferences
Most (16/20 [80.0%]) physicians who had prescribed some biosimilars and practice managers did not notice clinical differences between the biosimilars and their respective reference products and did not perceive any clinical differences. A minority (4/20 [20.0%]) were either unsure whether there were any clinical differences or felt that there were differences but that they were not significant.
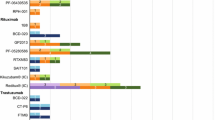
When payers were probed for the formulary/medical policy coverage for biosimilars of trastuzumab, rituximab, and bevacizumab, more than 70.0% (for each, 9/12) reported that the reference products were in the non-preferred position (Table 2). The nine MCOs that placed reference products in non-preferred positions implemented a common policy to favor biosimilars among all three products. All 12 MCOs provided coverage, even if not preferred, of all biosimilars for these three reference products. Three of the nine MCOs preferred specific biosimilars for trastuzumab (one preferred one biosimilar, one preferred two biosimilars, and one preferred three biosimilars). One MCO preferred one biosimilar for rituximab and bevacizumab. The IDNs differed from the MCOs, with most IDNs limiting their biosimilar preferences for inventory management considerations. Overall, for trastuzumab biosimilars, trastuzumab-anns and trastuzumab-qyyp were most frequently in a preferred position. Rituximab-pvvr and bevacizumab-awwb were in a preferred position most often among biosimilars of rituximab and bevacizumab, respectively.
3.3 Factors that Contributed to Positive Biosimilar Perceptions
In open-ended responses, most physicians considered cost effectiveness (14/17 [82.4%]) and efficacy (13/17 [76.5%]) as the primary factors that influenced their positive views of biosimilar adoption (Table 3). Although the FDA has not yet approved any oncology biosimilar products as interchangeable, hypothetically having FDA interchangeability designation (12/17 [70.6%]), followed by safety (8/17 [47.1%]), were the next most commonly reported factors that would influence positive views of biosimilar adoption. Other factors that were spontaneously mentioned more than once were regulatory approval and different clinical and commercial features, including extrapolation (i.e., when a biosimilar is approved for use in a reference product indication when that indication was not included in the biosimilar clinical trial), manufacturer country, and the number of biosimilars on the market for a specific category.
In open-ended responses, nearly two-thirds (13/20 [65.0%]) of the payers interviewed spontaneously reported that the cost of a biosimilar, relative to its reference product, was a major factor influencing their positive perceptions of biosimilar adoption (Table 3). This was followed in importance by physician comfort with prescribing biosimilars (10/20 [50.0%]) and number of marketed biosimilars in that drug class (9/20 [45.0%]). Other factors noted by four or more (≥ 20.0%) payers included efficacy, safety, clinical guidelines, hypothetical FDA interchangeability designation, and patient comfort with taking biosimilars.
Financial considerations, particularly cost savings/pricing (10/15 [66.7%]), were strongly emphasized by most physicians as influencing support for biosimilar use in their practice (Table 4). Although cost was the central consideration, physicians believed it was crucial for manufacturers to provide the same types of support services for biosimilars that they ordinarily provide for the reference products, including educational resources and product support (7/15 [46.7%]), copay assistance and patient support programs (6/15 [40.0%]), and handouts with biosimilar details and formulation information (2/15 [13.3%]). The remaining themes of importance to physicians focused on confidence in biosimilars, such as supporting efficacy/safety data and FDA approval (6/15 [40.0%]), and the reliability and availability of the biosimilar product supply (4/15 [26.7%]).
More than half (11/20 [55.0%]) of the payers interviewed spontaneously said cost benefits/contracting for biosimilars contributed the most towards decisions on formulary inclusion (Table 4). Formulary decisions were also influenced by whether extrapolation to other indications was allowed (6/20 [30.0%]), by FDA approval and clinical guidelines (5/20 [25.0%]), and by the availability of safety/efficacy data on biosimilars (3/20 [15.0%]). For most IDNs (7/8 [87.5%]), third-party payers’ reimbursement choices significantly influenced their own decision-making process regarding whether they added biosimilars to the formulary and which biosimilars they added. The other factors influencing formulary decisions for payers included physician comfort with prescribing biosimilars (3/20 [15.0%]) as well as trusted manufacturer and administrative issues (2/20 [10.0%]).
3.4 Clinical and Operational Aspects of Biosimilars
Physicians and practice managers almost unanimously reported perceiving a similar or the same degree of challenge with clinical aspects of using biosimilars and the respective reference products (Table 5). One physician reported that a few features were more challenging because of a perception that biosimilars are slightly inferior to the reference products. However, this physician’s comment was based on their experience with biosimilars other than those of trastuzumab, rituximab, or bevacizumab.
Most physicians were comfortable with practice protocols that allowed for the pharmacist or financial administrative team to select which biosimilar was best for the patient, given the financial benefit to the patient and practice, as well as the preference of the patient’s insurance company. Only one physician was uncomfortable with this approach because of a belief that automatic substitution removes a degree of the physician’s influence over patient care.
Although perceptions of operational challenges varied among physicians and practice managers, the majority perceived all assessed operational aspects of biosimilars to be as challenging or less challenging than those of the reference products (Table 5). The operational aspects that physicians and practice managers most often perceived as being more challenging with biosimilars included pre-certification/prior authorization (6/20 [30.0%]), inventory management (6/20 [30.0%]), electronic health records (5/20 [25.0%]), and the need for staff education (4/20 [20.0%]). Physicians and practice managers who perceived more challenging operational differences in pre-certification/prior authorization noted that payers’ systems are often not initially updated with new biosimilars or that physicians might have to change the order after learning a different biosimilar is preferred; likewise, prior authorization staff need to stay highly aware of all the changes that occur with this process. Among those who perceived inventory management as a greater challenge with biosimilar use, this was largely because of the need to track and manage multiple biosimilars for the same reference product. Of those who reported that electronic health records raised challenges with biosimilar use, the specific issues related to the software’s inability to incorporate payer-specific biosimilar decision criteria and difficulty distinguishing biosimilars based on their generic names and four-letter suffixes. Lastly, greater challenges with biosimilar use were perceived by some respondents because of the extra steps and support needed to educate clinical and administrative staff on biosimilars.
3.5 Step Therapy and Use of Biosimilars in Treatment-Naïve Patients
Physicians and practice managers were asked to estimate the percentage of patients that received biosimilars in a naïve setting versus being switched from the reference product. Of all their patients who received biosimilars, most were naïve to the reference product (10/15 [66.7%], 14/16 [87.5%], and 12/15 [80.0%] reported > 50% biosimilar utilization for patients naïve to trastuzumab, rituximab, and bevacizumab reference products, respectively). Reference product to biosimilar switching was less common and varied across practices, with physicians most often reporting that reference product to biosimilar switching occurred for < 25% of their patients who received biosimilars (7/15 [46.7%], 9/16 [56.3%], and 9/15 [60.0%] for trastuzumab, rituximab, and bevacizumab reference products, respectively). Biosimilar-to-biosimilar switching was not investigated, even though a given physician may have prescribed more than one biosimilar.
Payers preferred biosimilars for treatment-naïve patients, and they planned to encourage biosimilar use by implementing step therapy in which treatment-naïve patients would receive a biosimilar and only receive the reference product if they were intolerant to treatment on the biosimilar. However, only two (10.0%) of the 20 payers interviewed reported that they were implementing switching policies for biosimilars among current patients using a reference product.
4 Discussion
The oncology treatment landscape has been evolving since the FDA’s approval of the first oncology biosimilar in 2017 [21]. Despite having a slow start, there are positive signs of growing biosimilar adoption in oncology, considering the cost savings benefits for providers, payers, and patients. The three recently launched oncology therapeutic biosimilars of bevacizumab, trastuzumab, and rituximab have achieved 42%, 38%, and 20% uptake, respectively, within their first year on the market according to a recent IQVIA report on biosimilar trends in the USA between 2020 and 2024, which is significantly higher and faster than that of prior biosimilars [1]. Our results support current trends [1], indicating that biosimilars are beginning to make headway in the USA.
However, challenges remain in terms of biosimilar adoption in the USA, including gaps in prescriber knowledge about biosimilars [17, 22, 23]. According to a survey conducted among 300 managed care and specialty pharmacy professionals, education about evidence from switching studies and FDA guidance on pharmacy-level substitution of reference products with biosimilars were the highest-rated strategies to overcome biosimilar adoption challenges in the USA [22]. Similarly, a focus group discussion that consisted of five managed care pharmacists and three physicians was conducted in 2019 in Boston [23]. This group identified major barriers for biosimilar adoption, including a lack of confidence in biosimilar interchangeability, a need for education about biosimilars, and administrative burdens preventing the prescription of biosimilars [23]. These hurdles to biosimilar usage were consistent with findings from prior studies [14, 16, 17], which highlighted the importance of education programs for key stakeholders and streamlining administrative processes to facilitate biosimilar prescription. However, it is worth noting that, as physicians’ exposure to biosimilars increases, so too do their perceptions and knowledge of biosimilars, and new strategies are being developed to streamline billing, coding, stocking, dispensing, and reimbursement processes for biosimilars, all of which may explain the more positive perceptions and experiences about biosimilars observed in the present study compared with those from studies when oncology mAb biosimilars were still relatively new to the US market [15,16,17].
Most physicians and practice managers interviewed believed biosimilar efficacy and safety to be similar or equivalent to that of the reference products—a belief that did not differ by practice setting—and reported being comfortable using biosimilars for all FDA-approved indications, including extrapolated indications. Our findings were consistent with those from a survey administered among US healthcare professionals, which found that 88% of respondents recognized FDA-approved biosimilars as safe and efficacious and 78% agreed that extrapolation across indications was also safe and effective [24]. Also, another study showed that approximately 95% of surveyed US community oncologists were very or somewhat confident that biosimilars were as safe and effective as their reference products [15]. A more recent survey of 602 specialists who regularly prescribed biologics found that physicians perceived biosimilars as equally safe and effective as the reference products [25]. Initial levels of skepticism and uncertainty expressed by physicians regarding the safety and efficacy of integrating biosimilars into oncology care have gradually diminished with their increased experience prescribing biosimilars and continuing education efforts [17, 26, 27]. Regarding awareness of other major topics related to biosimilars, such as the FDA approval process and definition of interchangeability, the level of understanding varied among the physicians interviewed, which aligns with some previous findings [8, 28, 29]. However, it is worth noting that HCPs interviewed in the present study all had some experience with biosimilars, so their views on biosimilars might not reflect those who have not yet been exposed to biosimilars.
Likewise, most interviewed payers shared a positive view of oncology biosimilars and perceived their safety and efficacy as comparable to those of the originator. Wilde et al. [25] reported that group purchasing organization leaders indicated strong confidence in the safety and efficacy of biosimilars. Payers’ positive perceptions of biosimilars have developed partially because of their observations of physicians’ comfort with prescribing biosimilars, the FDA’s approval, and the use of “totality of evidence” to evaluate biosimilars. Sparse real-world evidence (RWE) to support the interchangeability of biosimilars was attributed to the late-adoption rates of biosimilars in oncology among MCO/PBMs, which highlights the need to generate RWE to maximize biosimilar uptake [30].
We found that most MCO/PBMs were using utilization management tools to encourage uptake of biosimilars, including use of a biosimilar rather than a reference product through their prior authorization process for patients not previously on the reference product (treatment naïve), although policies for implementing biosimilar use among patients who were already on a reference product were rare. Formulary exclusion and step therapy have been proposed as viable strategies to control spending and eliminate some issues associated with differential cost sharing by promoting biosimilar uptake [19]. Notably, we found that the majority of the interviewed payers reported non-preferred formulary positions for reference products, which was much higher than that reported by Chambers et al. [31], given only 14% of the US commercial health plans granted the biosimilar preferred coverage. However, consistent with the current study, all the health plans offered on-par coverage for the only two oncology therapeutic biosimilars (bevacizumab-awwb and trastuzumab-anns) included in Chambers et al. [31]. As the study by Chambers et al. [31] was conducted when very few oncology therapeutic biosimilars were available on the market (bevacizumab-awwb and trastuzumab-anns), and the reimbursement landscape keeps evolving as more oncology biosimilars enter the US market (the present study included all nine available oncology therapeutic biosimilars), further studies with larger sample sizes of US commercial payers are needed to understand how oncology biosimilars are covered relative to reference products. Payers’ use of such policies was aligned with what we heard from the physicians we interviewed, as nearly 70% of physicians reported using biosimilars in over half of their treatment-naïve patients, although physicians did not mention any preference for prescribing biosimilars for more versus less medically complicated patients, as was seen in another study [16]. Our results were supported by a survey conducted by the nonpartisan and objective research organization NORC at the University of Chicago, which showed that 49% of physicians reported that they were very likely to prescribe biosimilars for new patients, compared with 31% who were very likely to prescribe biosimilars for patients currently on reference products [25]. Payers’ apprehension about switching is probably due to theoretical concerns that transitioning from one biologic to another may result in loss of efficacy or adverse events [32]. These concerns could likely be mitigated if more switching data can be provided in oncology, as shown in other therapeutic areas [33].
Notably, cost effectiveness was the factor most frequently cited as influencing the usage of biosimilars among HCPs and payers in the current study, which is supported by a prior survey of US academic oncologists that noted cost difference as the second most important deciding factor when prescribing a biosimilar [16]. Some physicians in our study agreed that the lower cost of biosimilars would likely result in improved access to cancer treatment with biologics. It is well-established that cancer-related financial toxicity (CRFT) is associated with an increased risk for medical noncompliance and delayed prescription refills and critical care, which can negatively affect clinical outcomes [34,35,36]. The introduction of biosimilars may help mitigate CRFT in patients with cancer [37]. Furthermore, integrating biosimilars into oncology care is consistent with the US movement to value-based care and accountable care, one component of which is efficiency in the delivery of care [38].
Along with the cost advantages of biosimilars, contracting was also recognized as an important factor driving biosimilar adoption and selection. Most MCO/PBMs reported that they covered all FDA-approved biosimilars, but IDNs had usually placed restrictions on the number of biosimilars they preferred, which was largely influenced by favorable contracting. Streamlining contracting was proposed as one of the strategies to overcome barriers to biosimilar adoption, given that organizational preferences for reference products could largely depend on contracting rebates [22]. Biosimilar contractual arrangement is advancing with innovative payment models, such as shared savings programs, to fully realize the saving potential of biosimilars [39]. On the other side, physicians in our study emphasized that they are generally not involved in the process of selecting which specific biosimilar a patient will receive for their treatment, which was a decision made at the practice level or depended on the patient’s insurance. Most physicians were comfortable with automatic substitution of a reference drug or a biosimilar product for another biosimilar product or the reference drug by the administrative team, but only one physician felt this approach was suboptimal. The authors of the aforementioned NORC survey reported that 75% of physicians were against automatic substitution by pharmacists, given the lack of knowledge about treatment decisions for the patients [25]. Future efforts are needed to develop and optimize state regulations of biosimilar substitution, leveraging the role of pharmacists in driving biosimilar uptake [40, 41].
Operationally, biosimilar implementation was considered similar to that of the reference products or any new products in most aspects, although respondents emphasized that continuing education for all stakeholders is critical to the successful adoption of biosimilars in oncology, which was aligned with prior studies [26, 28, 42]. Other implementation considerations mentioned by some of our respondents included manufacturer supply chain security; proper drug storage, handling, and tracking; and inventory management with more biosimilars entering the market; pharmacovigilance requirements; and enhanced electronic medical record systems, which were also commonly recognized in previous studies [32, 43, 44]. However, none of those factors was cited as an obstacle for biosimilar adoption in our study.
4.1 Limitations
The use of qualitative methodology enables an in-depth understanding of the real-world experiences and perceptions of oncology biosimilar adoption among physicians, practice managers, and payers. Yet, given the small samples inherent to qualitative research, results may not generalize to the broader stakeholders in the USA. For example, the interviewees in the current study all had experience with oncology biosimilars, so our findings might not represent the same level of knowledge, awareness, and perceptions of those with no or limited experiences with oncology biosimilars. However, the primary objective of the present study did not focus on generalizability; rather, we sought to gain a deeper understanding of the trends in patterns of utilization and real-world implementation success of biosimilar usage as the oncology treatment landscape changes. Research indicates that saturation of responses (i.e., the point at which interviewing additional respondents no longer yields any new concepts or themes) is generally attained by the 12th interview [20]. To reflect the potential for different general themes to emerge among physicians/practice managers and payers, 20 respondents per cohort were included in the current study, which enabled response saturation to be reached with each cohort. Second, we acknowledge that patients, who were not included in the present study, play a pivotal role in successful biosimilar adoption. Lastly, self-selection bias was a possibility, as participation in the study was voluntary.
5 Conclusion
Physicians, practice managers, and payers in the USA with experience with oncology biosimilars had favorable views of biosimilars, particularly for use among treatment-naïve patients. A framework for integrating biosimilars into oncology practice is underway, which is primarily driven by insurance coverage policy, contracting, and cost benefits. The findings from this study may be used to guide the implementation of appropriate operational efforts to ensure successful adoption of biosimilars in the USA.
References
The IQVIA Institute, Biosimilars in the United States 2020–2024; 2020. https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2020-2024.
Gottlieb S. Remarks from FDA Commissioner Scott Gottlieb, M.D., as prepared for delivery at the Brookings Institution on the release of the FDA’s Biosimilars Action Plan; 2018. https://www.fda.gov/news-events/press-announcements/remarks-fda-commissioner-scott-gottlieb-md-prepared-delivery-brookings-institution-release-fdas.
Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3.
Yang J, et al. Does biosimilar bevacizumab offer affordable treatment options for cancer patients in the USA? A budget impact analysis from US commercial and medicare payer perspectives. Appl Health Econ Health Policy. 2021;19:605–18.
U.S. Food and Drug Administration, FDA-Approved Biosimilar Products. U.S. Food & Drug Administration (2020). https://www.fda.gov/drugs/biosimilars/biosimilar-product-information.
Zhai MZ, Sarpatwari A, Kesselheim AS. Why are biosimilars not living up to their promise in the US? AMA J Ethics. 2019;21(8):E668-678.
Dalpoas SE, et al. Barriers to biosimilar utilization in the United States. Am J Health Syst Pharm. 2020;77(23):2006–14.
Leonard E, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–12.
Cohen H, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160–72.
Bonovas S, Peyrin-Biroulet L, Danese S. Clinical development of biologicals and biosimilars—safety concerns. Expert Rev Clin Pharmacol. 2017;10(6):567–9.
Boccia R, et al. Can biosimilars help achieve the goals of US health care reform? Cancer Manag Res. 2017;9:197–205.
Sarnola K, et al. Physicians’ perceptions of the uptake of biosimilars: a systematic review. BMJ Open. 2020;10(5): e034183.
Barsell A, Rengifo-Pardo M, Ehrlich A. A survey assessment of US dermatologists’ perception of biosimilars. J Drugs Dermatol. 2017;16(6):612–5.
Felix AE, et al. Barriers to market uptake of biosimilars in the US. Generics Biosimilars Initiat J. 2014;3(3):108–15.
Chadi N, et al. Community oncologists’ perception and acceptance of biosimilars in oncology. J Clin Pathways. 2018;4(4):43–7.
Cook JW, et al. Academic oncology clinicians’ understanding of biosimilars and information needed before prescribing. Ther Adv Med Oncol. 2019;11:1758835918818335.
Kolbe AR, et al. Physician understanding and willingness to prescribe biosimilars: findings from a US national survey. BioDrugs. 2021;35(3):363–72.
Sarpatwari A, et al. The US biosimilar market: stunted growth and possible reforms. Clin Pharmacol Ther. 2019;105(1):92–100.
Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34(2):294–301.
Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82.
Food and Drug Administration (FDA). FDA approves first biosimilar for the treatment of cancer; 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-first-biosimilar-treatment-cancer.
Greene L, et al. Strategies for overcoming barriers to adopting biosimilars and achieving goals of the biologics price competition and innovation act: a survey of managed care and specialty pharmacy professionals. J Manag Care Spec Pharm. 2019;25(8):904–12.
Edgar BS, et al. Overcoming barriers to biosimilar adoption: real-world perspectives from a national payer and provider initiative. J Manag Care Spec Pharm. 2021;27:2376–1032.
Herndon K, et al. Biosimilar Perceptions among healthcare professionals and commercial medical benefit policy analysis in the United States. BioDrugs. 2021;35(1):103–12.
Wilde S, et al. Understanding stakeholder perception of biosimilars. NORC at the University of Chicago; 2021. https://www.norc.org/PDFs/Biosimilars/20210405_AV%20-%20NORC%20Biosimilars%20Final%20Report.pdf.
Ismailov RM, Khasanova ZD. Biosimilar knowledge among oncology/hematology team members in Colorado, USA: an educational initiative and follow-up survey. BioDrugs. 2018;32(5):499–506.
Camacho LH. Current status of biosimilars in oncology. Drugs. 2017;77(9):985–97.
Ismailov RM, Khasanova ZD, Gascon P. Knowledge and awareness of biosimilars among oncology patients in Colorado, USA. Future Oncol. 2019;15(22):2577–84.
North American Center for Continuing Medical Education, CME survey: biosimilars; 2013. https://www.naccme.com/sites/naccme.com/files/biosimilar-survey-results.pdf.
Nava-Parada P, Shelbaya A, Nabhan C. Rituximab biosimilars in hematologic malignancies: the need for a real-world approach. Future Oncol. 2020;16(26):2017–27.
Chambers JD, et al. Coverage for biosimilars vs reference products among US commercial health plans. JAMA. 2020;323(19):1972–3.
Campen CJ. Integrating biosimilars into oncology practice: implications for the advanced practitioner. J Adv Pract Oncol. 2017;8(7):688–99.
Cohen HP, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463–78.
Knight TG, et al. Financial toxicity in adults with cancer: adverse outcomes and noncompliance. J Oncol Pract. 2018. https://doi.org/10.1200/JOP.18.00120.
Zafar SY, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–90.
Coughlin SS, et al. Financial distress among breast cancer survivors. Curr Cancer Rep. 2020;2(1):48–53.
Saleem T, et al. Biosimilars as a future, promising solution for financial toxicity: a review with emphasis on bevacizumab. Cureus. 2020;12(7): e9300.
MedPAC. Assessing payment adequacy and updating payments in fee-for-service Medicare in Report to the Congress: Medicare Payment Policy; 2018.
Manolis CH, et al. Biosimilars: opportunities to promote optimization through payer and provider collaboration. J Manag Care Spec Pharm. 2016;22(9 Suppl):S3–9.
Stevenson JG, et al. Biosimilars: practical considerations for pharmacists, pp. 1542–6270 (Electronic).
Sacks CA, et al. Assessment of variation in state regulation of generic drug and interchangeable biologic substitutions. JAMA Intern Med. 2021;181(1):16–22.
Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T Committees, pp. 1052–1372 (Print).
Griffith N, et al. Formulary selection criteria for biosimilars: considerations for US health-system pharmacists. Hosp Pharm. 2014;49(9):813–25.
Tinsley SM, et al. Potential of biosimilars to increase access to biologics: considerations for advanced practice providers in oncology. J Adv Pract Oncol. 2018;9(7):699–716.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Pfizer Inc. The sponsor’s role in study design, collection, analysis, and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication was limited to the involvement of two of the manuscript authors who are employees of the sponsor.
Conflict of interest
Dr. Yang and Dr. Shelbaya are employees of and hold stock in Pfizer Inc. Ms. Blinzler, Mr. Lankin, Dr. Vijayakumar, and Dr. Maculaitis are employees of Kantar Health, which received funding from Pfizer for conducting and reporting on the study.
Ethics approval
The current study utilized deidentified qualitative interview data. The study protocol was reviewed and deemed exempt by Pearl Institutional Review Board (protocol: 20-KANT-236) according to FDA 21 CFR 56.104 and 45CFR46.104(b)(2). The study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
To be eligible to participate in this study, respondents had to provide informed consent, which was collected from each respondent electronically.
Consent to publish
The informed consent statement indicated that data collected from respondents would be reported along with the data of other participants and advised respondents that they could not be personally identified with their responses. Thus, when providing their consent to participate in the study, respondents likewise consented to the publication of the study data.
Availability of data and material
Data and study materials can be made available for non-commercial use upon reasonable request to the corresponding author.
Code availability
Codes can be made available for non-commercial use upon reasonable request to the corresponding author.
Author contributions
Jingyan Yang: Conceptualization, methodology, writing of the original draft, and revisions. Joshua Lankin: Methodology, interview, modeling, and manuscript review and revisions. Kelly Blinzler: Conceptualization, methodology, interview, supervision, and manuscript review and revisions. Sapna Vijayakumar: Modeling and manuscript review and revisions. Martine C. Maculaitis: Supervision and manuscript review and revisions. Ahmed Shelbaya: Conceptualization, supervision, and manuscript review and revisions. All authors approved of the final version of the manuscript to be published and agreed to be accountable for all aspects of the work.
Prior peer-reviewed presentation
A portion of the results reported in this manuscript was presented at the virtual International Society for Pharmacoeconomics and Outcomes Research (ISPOR) annual conference, 17–20 May 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, J., Blinzler, K., Lankin, J. et al. Evolving Perceptions, Utilization, and Real-World Implementation Experiences of Oncology Monoclonal Antibody Biosimilars in the USA: Perspectives from Both Payers and Physicians. BioDrugs 36, 71–83 (2022). https://doi.org/10.1007/s40259-021-00509-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-021-00509-3