Abstract
Background and aims
Immunogenicity with formation of anti-drug antibodies (ADA) to biologics is an important reason for treatment failure in inflammatory bowel disease (IBD). Our aim was to assess the rate of ADA, the effect of combination therapy with immunomodulators on ADA and the influence of ADA on efficacy and safety of biologics for IBD treatment.
Methods
MEDLINE, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from inception to April 2020 for trials of biologics that assessed immunogenicity. The overall certainty of evidence was evaluated using Grading of Recommendations, Assessment, Development and Evaluations (GRADE). The primary outcome was rate of ADA. Secondary outcomes included efficacy and safety outcomes among patients with detectable versus undetectable ADA. For dichotomous outcomes, pooled risk ratios (RR) and 95% confidence intervals (CI) were calculated.
Results
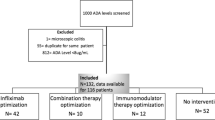
Data from 68 studies were analyzed and 33 studies (5850 patients) were included in the meta-analysis. Pooled ADA rates for biologic monotherapy were 28.0% for infliximab, 7.5% for adalimumab, 3.8% for golimumab, 10.9% for certolizumab, 6.2% for ustekinumab and 16.0% for natalizumab. Pooled ADA rates were 8.4% for vedolizumab and 5.0% for etrolizumab for combo- and monotherapy combined. In all biologics, ADA rates were underestimated by use of drug-sensitive ADA assays and higher dose and/or frequency. ADA rate was significantly reduced in patients treated with combination therapy for infliximab (RR 0.52; 95% CI 0.44–0.62), adalimumab (RR 0.31; 95% CI 0.14–0.69), golimumab (RR 0.29; 95% CI 0.10–0.83), certolizumab pegol (RR 0.30; 95% CI 0.14–0.67) and natalizumab (RR 0.20; 95% CI 0.11–0. 39). ADA to infliximab were associated with lower clinical response rates (RR 0.75; 95% CI 0.61–0.91) and higher rates of infusion reactions (RR 2.36; 95% CI 1.85–3.01).
Conclusions
Differences in analytical methods to detect ADA hamper comparison of true ADA rates across biologics in IBD. Use of combination therapy with immunomodulators appeared to reduce ADA positivity for most biologics. For infliximab, ADA were associated with reduced drug efficacy and increased adverse events.







Similar content being viewed by others
References
van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut. 2014;63(1):72–9.
Bots SJA, Hoekman DR, Benninga MA, Ponsioen CY, D’Haens GR, Lowenberg M. Patterns of anti-TNF use and associated treatment outcomes in inflammatory bowel disease patients: results from an analysis of Dutch health insurance claims data. Neth J Med. 2017;75(10):432–42.
Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol (Baltimore, Md: 1950). 1997;159(12):6276–82.
Masuda H, Iwai S, Tanaka T, Hayakawa S. Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative colitis, particularly in patients with inactive phase. J Clin Lab Immunol. 1995;46(3):111–23.
Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles-Komar JM, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3(6):535–45.
Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol (Baltimore, Md: 1950). 1994;152(7):3282–93.
Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864–75.
Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut. 2013;62(8):1122–30.
Baert F, Noman M, Vermeire S, Van Assche G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–8.
Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137(5):1628–40.
O’Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20(1):1–6.
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95.
Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392-400.e3.
Adedokun OJ, Gunn GR, Leu JH, Gargano C, Xu Z, Sandborn WJ, et al. Immunogenicity of golimumab and its clinical relevance in patients with ulcerative colitis. Inflamm Bowel Dis. 2019;25(9):1532–40.
Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53.
Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology. 2003;124(4):917–24.
Ben-Horin S, Waterman M, Kopylov U, Yavzori M, Picard O, Fudim E, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(4):444–7.
Strik AS, van den Brink GR, Ponsioen C, Mathot R, Lowenberg M, D’Haens GR. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(8):1128–34.
Hart MH, de Vrieze H, Wouters D, Wolbink GJ, Killestein J, de Groot ER, et al. Differential effect of drug interference in immunogenicity assays. J Immunol Methods. 2011;372(1–2):196–203.
Steenholdt C, Ainsworth MA, Tovey M, Klausen TW, Thomsen OO, Brynskov J, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit. 2013;35(4):530–8.
Higgins JPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane; 2020. www.training.cochrane.org/handbook.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Bots S, Vande Casteele N, Brandse JF, Lowenberg M, Feagan BG, Sandborn WJ, et al. Antibody development against biologic agents used for the treatment of inflammatory bowel disease and antibody prevention with immunosuppressives. Cochrane Database Syst Rev. 2016(5).
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. 2017. www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56(9):1226–31.
Oh EH, Ko DH, Seo H, Chang K, Kim GU, Song EM, et al. Clinical correlations of infliximab trough levels and antibodies to infliximab in South Korean patients with Crohn’s disease. World J Gastroenterol. 2017;23(8):1489–96.
Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147(6):1296-307 e5.
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59(1):49–54.
Van Stappen T, Vande Casteele N, Van Assche G, Ferrante M, Vermeire S, Gils A. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67(5):818–26.
Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016;150(7):1568–78.
Ye BD, Pesegova M, Alexeeva O, Osipenko M, Lahat A, Dorofeyev A, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393(10182):1699–707.
Roblin X, Williet N, Boschetti G, Phelip JM, Del Tedesco E, Berger AE, et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut. 2020;69(7):1206–12.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96-109.e1.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95 (quiz e14–e15).
Adedokun OJ, Xu Z, Marano CW, Strauss R, Zhang H, Johanns J, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis. 2017;11(1):35–46.
Sandborn W, Dubinsky M, Kosutic G, Parker G, Spearman M, Hasan I, et al. Incidence of anti-drug antibodies in Crohn’s disease patients during 5 years of certolizumab pegol therapy. Inflamm Bowel Dis. 2016;22:S41.
Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–38.
Sandborn WJ, Wolf DC, Kosutic G, Parker G, Schreiber S, Lee SD, et al. Effects of transient and persistent anti-drug antibodies to certolizumab pegol: longitudinal data from a 7-year study in Crohn’s disease. Inflamm Bowel Dis. 2017;23(7):1047–56.
Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
Wyant T, Yang L, Lirio R, Rosario M. Long-term immunogenicity of vedolizumab in ulcerative colitis and Crohn’s disease (GEMINI Programme). J Crohns Colitis. 2019;13(Supplement 1):S331.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6(12):1370–7.
Sandborn WJ, Baert F, Danese S, Krznaric Z, Kobayashi T, Yao X, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562–72.e12.
Adedokun OJ, Xu Z, Marano C, O’Brien C, Szapary P, Zhang H, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis: ustekinumab PK and exposure-response in UC. Clin Gastroenterol Hepatol. 2019;06:06.
Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14.
Vermeire S, O'Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384(9940):309–18.
Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–1329 e3.
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9.
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–85.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352(24):2499–507.
Sandborn WJ, Baert F, Danese S, Krznaric Z, Kobayashi T, Yao X, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562–72. e12.
Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OO, Hanauer SB, McColm J, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357(3):239–50.
Steenholdt C, Bendtzen K, Brynskov J, Thomsen OO, Ainsworth MA. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol. 2014;109(7):1055–64.
Brandse JF, Mould D, Smeekes O, Ashruf Y, Kuin S, Strik A, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(4):650–60.
Papamichael K, Chachu KA, Vajravelu RK, Vaughn BP, Ni J, Osterman MT, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15(10):1580-8.e3.
Schreiber S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, Pukitis A, Horynski M, Farkas K, Kierkus J, Kowalski M. Noninferiority of novel subcutaneous infliximab (ct-p13) to intravenous infliximab (ct-p13) in patients with active Crohn’s disease and ulcerative colitis: week 30 results from a multicentre, randomised controlled pivotal trial. United Eur Gastroenterol J. 2019;7(10):1412.
Ungar B, Chowers Y, Yavzori M, Picard O, Fudim E, Har-Noy O, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63(8):1258–64.
Roblin X, Marotte H, Leclerc M, Del Tedesco E, Phelip JM, Peyrin-Biroulet L, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015;9(7):525–31.
Roblin X, Vérot C, Paul S, Duru G, Williet N, Boschetti G, et al. Is the Pharmacokinetic Profile of a First Anti-TNF Predictive of the Clinical Outcome and Pharmacokinetics of a Second Anti-TNF? Inflammatory bowel diseases. 2018;24(9):2078–85.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010;69(5):817–21.
Casanova MJ, Chaparro M, Garcia-Sanchez V, Nantes O, Leo E, Rojas-Feria M, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol. 2017;112(1):120–31.
Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology. 2015;149(7):1716–30.
García Beloso N, Altabás González I, Samartín Ucha M, Gayoso Rey M, De Castro Parga ML, Salgado Barreira Á, et al. Switching between reference adalimumab and biosimilars in chronic immune-mediated inflammatory disease: a systematic literature review. Br J Clin Pharmacol. 2021 Oct 8. https://doi.org/10.1111/bcp.15101 [Online ahead of print].
Albshesh A, Ben-Horin S. CT-P13: a review on a biosimilar to infliximab in the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2019;19(10):971–8.
Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. 2020;158(1):189–99.
Steenholdt C, Svenson M, Bendtzen K, Thomsen OO, Brynskov J, Ainsworth MA. Severe infusion reactions to infliximab: aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(1):51–8.
West R, Woude C, Hansen B, Felt-Bersma R, Tilburg A, Drapers J, et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn's disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2004;20(11–12):1329–36. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/054/CN-00514054/frame.html.
Pecoraro V, De Santis E, Melegari A, Trenti T. The impact of immunogenicity of TNFalpha inhibitors in autoimmune inflammatory disease. A systematic review and meta-analysis. Autoimmun Rev. 2017;16(6):564–75.
Dreesen E, Van Stappen T, Ballet V, Peeters M, Compernolle G, Tops S, et al. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn’s disease who lose clinical response. Aliment Pharmacol Ther. 2018;47(3):346–55.
Roblin X, Boschetti G, Williet N, Nancey S, Marotte H, Berger A, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open-label, prospective and randomised clinical trial. Aliment Pharmacol Ther. 2017;46(2):142–9.
Schaeverbeke T, Truchetet ME, Kostine M, Barnetche T, Bannwarth B, Richez C. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology (Oxford). 2016;55(2):210–20.
Acknowledgements
We would like to thank John MacDonald (Alimentiv Inc, London, Ontario, Canada) for his assistance in the writing process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant support
NVC holds a Research Scholar Award from the American Gastroenterological Association. WJS and NVC are supported in part by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Disclosures
SB has served as speaker for Abbvie, Merck, Sharp & Dome, Takeda, Jansen Cilag, Pfizer and Tillotts. CEP is an employee of Robarts Clinical Trials, Inc. JFB has served as speaker for Abbvie, Merck, Sharp & Dome, Takeda and Tillotts. ML has served as speaker and/or principal investigator for Abbvie, Celgene, Covidien, Dr Falk, Ferring Pharmaceuticals, Gilead, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Protagonist Therapeutics, Receptos, Takeda, Tillotts, Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Achmea Healthcare and ZonMW. BGF has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGeniX and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, ActoGeniX, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, gIcare Pharma Inc. and Sigmoid Pharma; and speaker bureau fees from UCB, AbbVie and J&J/Janssen. WJS has received research grants from Abbvie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, Glaxo Smith Kline, Janssen, Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma; consulting fees from Abbvie, Abivax, AdMIRx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), BeiGene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, Zealand Pharma; stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Biosciences; and is an employee at Shoreline Biosciences. Spouse: Iveric Bio (consultant, stock options); Progenity (stock); Oppilan Pharma (consultant, stock options); Prometheus Biosciences (employee, stock, stock options); Prometheus Laboratories (stock, stock options, consultant); Ventyx Biosciences (stock, stock options); Vimalan Biosciences (stock, stock options). VJ has received scientific advisory board fees from Janssen, Takeda, Pfizer, Sandoz, Abbvie and Merck; and speaker bureaux fees from Takeda, Ferring, Janssen, Pfizer and Shire. GDH has served as advisor for Abbvie, Ablynx, Allergan, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Covidien/Medtronic, Ferring, Dr Falk Pharma, Eli Lilly, enGene, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Hospira/Pfizer, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp & Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prometheus laboratories/Nestle, Progenity, Protagonist, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, TiGenix, Tillotts, Topivert, Versant and Vifor; received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp & Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor. NVC has received research grants and personal fees from R-Biopharm, Takeda and UCB; and personal fees from Alimentiv, Inc. (formerly Robarts Clinical Trials, Inc.), Celltrion and Prometheus.
Availability of data and material
All data will be made available.
Writing assistance
No funding was received for writing assistance.
Author contributions
Study concept and design: SB, GDH, NVC; acquisition of data: SB, JFB, NVC; analysis and interpretation of data: SB, CEP, NVC; draft of the manuscript: SB, CEP, NVC; critical revision of the manuscript for important intellectual content: SB, JFB, ML, BGF, WJS, RK, VJ, GDH, NVC; study supervision: GDH, NVC. All authors have approved the final version of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bots, S.J., Parker, C.E., Brandse, J.F. et al. Anti-Drug Antibody Formation Against Biologic Agents in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioDrugs 35, 715–733 (2021). https://doi.org/10.1007/s40259-021-00507-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-021-00507-5