Abstract
Type 2 diabetes is one of the major chronic diseases accounting for a substantial proportion of disease burden in Western countries. The majority of the burden of type 2 diabetes is attributed to environmental risks and modifiable risk factors such as lifestyle. The environment we live in, and changes to it, can thus contribute substantially to the prevention of type 2 diabetes at a population level. The ‘exposome’ represents the (measurable) totality of environmental, i.e. nongenetic, drivers of health and disease. The external exposome comprises aspects of the built environment, the social environment, the physico-chemical environment and the lifestyle/food environment. The internal exposome comprises measurements at the epigenetic, transcript, proteome, microbiome or metabolome level to study either the exposures directly, the imprints these exposures leave in the biological system, the potential of the body to combat environmental insults and/or the biology itself. In this review, we describe the evidence for environmental risk factors of type 2 diabetes, focusing on both the general external exposome and imprints of this on the internal exposome. Studies provided established associations of air pollution, residential noise and area-level socioeconomic deprivation with an increased risk of type 2 diabetes, while neighbourhood walkability and green space are consistently associated with a reduced risk of type 2 diabetes. There is little or inconsistent evidence on the contribution of the food environment, other aspects of the social environment and outdoor temperature. These environmental factors are thought to affect type 2 diabetes risk mainly through mechanisms incorporating lifestyle factors such as physical activity or diet, the microbiome, inflammation or chronic stress. To further assess causality of these associations, future studies should focus on investigating the longitudinal effects of our environment (and changes to it) in relation to type 2 diabetes risk and whether these associations are explained by these proposed mechanisms.
Graphical abstract

Similar content being viewed by others

Introduction
Type 2 diabetes is a major chronic disease burden in Western countries, which is estimated to affect 642 million people worldwide by 2040 [1]. Its increasing prevalence can be explained by non-modifiable factors, such as the ageing of the population, and modifiable factors like overweight/obesity and unhealthy lifestyle habits [1, 2]. Large prevention trials show that the risk of type 2 diabetes is reduced by approximately 50% by lifestyle modification in high-risk populations [3]. However, translation of such interventions into real-life and less-controlled settings is challenging [4], with the overall risk reduction falling to just 15% after 6 years [5]. One reason for this might be that many interventions do not sufficiently account for the context or living environment of the individual and rely primarily on the cognitive capacities and intrinsic motivation of those targeted [6]. Therefore, interventions may be more impactful if an individual’s environment is accounted for.
The human genome project revolutionised our understanding of the genetic origins of disease. Genome-wide association studies estimate that genetic variation solely explains 15–20% of the burden of type 2 diabetes [7], although other studies show an estimated genetic contribution of around 45%, albeit with a wide range [8]. Nevertheless, a large part of the burden of type 2 diabetes is attributed to modifiable and/or environmental risk factors and their interactions with our genetic make-up. Indeed, population attributable fractions range from 10% for smoking to 48% for obesity [9]. However, many of the environmental drivers of type 2 diabetes remain unknown, hampering the development of effective prevention programmes. One reason for the lack of understanding of these environmental drivers is the lack of good measures of our ‘environment’, both in coverage (the number of environmental factors that can be quantified) and resolution (the quality with which the proxies capture true exposure).
To address the imbalance of our abilities to measure environmental factors compared with genetic factors, the term ‘exposome’ was coined in 2005 [10]. The ‘exposome’ represents the (measurable) totality of environmental, i.e. nongenetic, drivers of disease [10]. Analyses of biological perturbations at different molecular levels, together with environmental measurements, should provide insights on the internal and external exposome contributors [11] (see the text box for a glossary of terms).
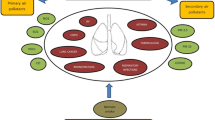
The external contributors to the exposome (i.e. the general external exposome) comprises aspects of the built environment (the physical space where we live and work), the social environment (the social relationships and social context in which groups of people live and interact, e.g. our socioeconomic position or social interactions), the physico-chemical environment (the chemical or physical agents present in our local area) and the lifestyle/food environment (the accessibility, availability, affordability and promotion of food and food retailers in our neighbourhood) (Fig. 1) [11]. The specific external exposome refers to individual exposures such as health behaviours. The internal exposome includes measurements at the epigenetic, transcriptome, proteome, microbiome or metabolome level to study either the exposures directly (e.g. non-targeted chemical screening [12]), the exposure imprints (e.g. biological imprints of smoking in the epigenome [13]), the potential of the body to combat environmental insults (i.e. allostatic load [14]) and/or the biology itself (Fig. 1) [15].
An overview of the three different domains of the exposome, adapted from Wild (2012) [112]. The general external exposome encompasses wider external influences of the living environment, whereas the specific external exposome reflects individual exposures. The internal exposome reflects how an individual’s biological processes and metabolism are impacted by these external exposures. This figure is available as part of a downloadable slideset
In this review, we describe the evidence for environmental risk factors of type 2 diabetes focusing on both the general external exposome and imprints of this on the internal exposome. Because aspects of the specific external exposome at the individual level, such as dietary patterns, physical activity and lifestyle modification programmes, have been reviewed elsewhere [3, 16], these factors will not be included. We provide a relative grading of the evidence indicated as stronger vs weaker evidence for a specific relationship. A stronger grade indicates an established association and is only provided when a systematic review or meta-analysis reported consistent results in at least three studies. A weaker grade indicates a suspected association and is provided when only incidental studies reported on the specific relationship or when a systematic review or meta-analysis did not yield consistent evidence. We conclude by providing methodological considerations and future perspectives for exposome research in the field of type 2 diabetes.
Environmental risk factors of type 2 diabetes
The food environment
The food environment encompasses the accessibility, availability, affordability and promotion of foods and food retailers [17]. The investigation of the food environment in relation to diet quality, obesity and chronic disease risk gained interest due to evidence of ‘food deserts’ in North America. Living in a ‘food desert’ with virtually no geographical access to food retailers has been associated with lower diet quality or disease outcomes [18]. Similarly, ‘food swamps’ represent environments where unhealthy food options outweigh healthy options.
Food environment research most frequently defines the food environment as geographical availability and accessibility to food retailers in the home neighbourhood, mainly operationalised as measures of the density of food retailers or distance to a specific food outlet, derived from underlying spatial data [19,20,21]. Exposure to unhealthy food retailers such as fast-food outlets has been associated with less healthy diets, obesity, increased insulin resistance, increased triacylglycerol concentration and type 2 diabetes, mainly in studies performed in the USA [22,23,24]. Despite these findings of a link between the food environment and type 2 diabetes, systematic reviews including over 15 studies report inconsistent findings or null results (Fig. 2) [20, 25, 26]. Subjective measures of exposure to food retailers, such as perceived availability or use of food retailers, are more consistently associated with type 2 diabetes risk than objective measures [27, 28]. Finally, interventions that change the exposure to food retailers are scarce, but a natural experiment in residential relocation after the 2011 earthquake and tsunami in Japan suggested that shortened distances to food outlets increased the risk of obesity and other cardiometabolic risk factors [29, 30].
An overview of the evidence of how elements of the food environment, built environment, physico-chemical environment and social environment are related to risk of type 2 diabetes, highlighting the potential pathways in which the three aspects of the exposome (general external, specific external and internal) interact. Evidence is indicated as stronger or weaker for each pathway. A stronger grade indicates an established association, based on consistent results in systematic reviews or meta-analyses of at least three studies. A weaker grade indicates a suspected association, where only incidental studies have reported the relationship, or where a systematic review or meta-analysis has not yielded consistent evidence. PM2.5, fine particulate matter 2.5 μm or less in diameter; T2D, type 2 diabetes. This figure is available as part of a downloadable slideset
The main reasons explaining these inconsistencies are: the over-simplistic definition of ‘exposure’, ignoring other environmental attributes like the social environment; the focus on the residential neighbourhood only, not accounting for exposure to the food environment at work or in transit; and the lack of insight into individuals’ behavioural interactions with the food environment [19,20,21, 26, 31, 32]. Changes in the spatial distribution of food outlets over time might also play a role, as several studies showed substantial changes in the food environment, mostly with increases of unhealthier food outlets, particularly in neighbourhoods with low socioeconomic position [33,34,35,36]. Whether such changes also affect the observed associations of the food environment with the risk of type 2 diabetes has been scarcely investigated. A study from Mexico that prospectively analysed changes in the food environment in relation to diabetes found that individuals living in neighbourhoods that experienced a decrease in the density of fruit and vegetable stores, and an increase in the density of convenience stores, had higher odds of diabetes compared with individuals living in neighbourhoods where these stores did not change [37].
The built environment
The built environment is defined as man-made characteristics of the physical environment in which people live, work and recreate, including buildings, streets, open spaces and infrastructure [38, 39]. The built environment is hypothesised to be associated with type 2 diabetes incidence primarily through physical activity-related pathways [40]. Indeed, meta-analyses consistently showed an established association of living in neighbourhoods with high walkability and green space with a 10–20% lower risk of type 2 diabetes [20, 25, 26] (Fig. 2), although mainly in studies performed in North America and Australia, while evidence for European countries is limited. Walkability of a neighbourhood is characterised by population density, land-use mix (i.e. heterogeneity of land uses in an area such as residential, industrial and natural) and connectivity (i.e. intersections). Other elements of the built environment in relation to risk of type 2 diabetes have not been investigated intensively. Six studies investigated availability of sports facilities in relation to risk of type 2 diabetes, but reported inconsistent results ranging from no association to a reduced risk with a higher availability [26]. A systematic review showed that interventions to increase physical activity by changes in the built environment generally improved levels of physical activity [41], but effects on type 2 diabetes have not been evaluated. Furthermore, a large Finnish cohort study of over 100,000 individuals showed that changes in residential greenness were associated with a 12% reduced risk of type 2 diabetes [42]. Finally, a meta-analysis showed an established association that urban dwellers, particularly in low- and middle-income countries, have a 40% increased risk of type 2 diabetes [26], but the underlying drivers of this association are not entirely clear. Apart from mainly lifestyle-related pathways, the built environment is characterised by interrelated factors [26, 43] that not only influence human behaviour, but can also directly affect disease risk (e.g. air pollution).
Physico-chemical environment
Air pollution has been documented to change endothelial function, trigger inflammation and insulin resistance, alter the gut microbiota and be associated with an elevated risk of hypertension [20, 44]. Recent meta-analyses showed an established association of an increased risk of type 2 diabetes with increased exposure to air pollution, with odds ratios (per 10 μg/m3 increase) ranging from 1.08–1.10 for particulate matter smaller than 2.5 μm (PM2.5), 1.10–1.12 for particulate matter smaller than 10 μm (PM10) and 1.05–1.08 for nitrogen dioxide (NO2) (Fig. 2) [20, 45]. Studies also suggested women may be more susceptible to exposure to pollution because it was posited that they may spend more time in and around the home than men [20]. Data on other pollutants are very limited, although a recent study showed sulphur dioxide as a risk factor [46]. There is inconsistency as to what pollutant is most correlated to type 2 diabetes, mainly because of the limited number and mixed results of multi-pollutant models [20].
Although only a few studies investigated the effect of light exposure on metabolic diseases [47], a relatively large study in Japanese care settings indicated that exposure to light at night could be associated with type 2 diabetes [48]. This association could potentially be explained by the consequent effects on lifestyle, particularly sleep disruption, which could result in elevated glucose levels [49].
Two meta-analyses showed an established association between higher residential, but not occupational, noise exposure and increased risk of type 2 diabetes (Fig. 2) [26, 50]. Noise can act as an environmental stressor that leads to insulin secretion and peripheral insulin sensitivity [50], but other lifestyle factors such as sleep may also be involved [50]. Finally, the associations of noise and light with type 2 diabetes could be confounded by other factors associated with urbanisation, such as air pollution.
Higher body temperature could negatively affect glucose metabolism by decreasing the brown adipose tissue mass and activity [51]. Experimental studies report high efficiency of cold exposure as a potential therapy for type 2 diabetes [52, 53]. Nevertheless, with the exception of one study reporting higher diabetes incidence with increasing annual outdoor temperature [54], evidence is limited for an association between ambient temperature and type 2 diabetes.
Finally, several meta-analyses established associations of chemical pollutants, such as persistent organic pollutants [55], pesticides [56] and heavy metals [57], with an increased risk of type 2 diabetes. These pollutants may originate from the complex chemical environment including occupational hazards, air or water pollution, and food contaminants. Many results suggest a stronger association between chemical pollutants and type 2 diabetes in women and individuals with overweight or obesity [57].
Social environment
The social environment is generally understood as the social relationships and social context in which groups of people live and interact [58]. Examples of social environment components include area-level deprivation, social capital, ethnic segregation and perceived safety. While individual-level social factors (e.g. education and income) are consistently associated with type 2 diabetes risk [59, 60], environmental social factors have recently received increased attention.
Systematic reviews consistently show that area-level socioeconomic deprivation is associated with an up to twofold increased incidence of type 2 diabetes (Fig. 2) [25, 61, 62]. In line with these studies, and suggesting causal relationship, natural experiments and studies of residential relocation show that moving to low deprivation neighbourhoods reduces HbA1c and risk of type 2 diabetes [42, 63, 64]. Despite growing evidence for the relationship between social capital and health [65], the few studies investigating social capital and type 2 diabetes incidence present mixed findings [61, 65,66,67,68]. Although the importance of good social networks in decreasing the risk of type 2 diabetes has been shown in large longitudinal studies, experimental studies are lacking [69]. Less is known about the impact of ethnic segregation on type 2 diabetes incidence. Nonetheless, a review by Kershaw and Pender (2016) concluded that ethnic segregation can influence the severity of type 2 diabetes but not its development [70]. Regarding discrimination, studies mostly investigated the impact of individually experienced, but not neighbourhood-level, discrimination on type 2 diabetes incidence [71]. Furthermore, only a few studies have investigated the impact of perceived crime within the neighbourhood on type 2 diabetes incidence, with some finding a significant association [72], and others indicating perceived crime as a weak moderator of amenities density and type 2 diabetes incidence [73].
Even though the evidence for an association between certain components of the social environment and risk of type 2 diabetes may be sparse or inconclusive, stronger links have been observed between social environment factors and lifestyle behaviours and obesity [70, 74,75,76,77,78], suggesting a plausible link with type 2 diabetes. Moreover, social environment factors are also hypothesised to influence type 2 diabetes incidence through chronic stress and inflammatory responses [79, 80].
Internal exposome
Although our internal exposome consists of proteins, lipids, metabolites and so on, the role of the metabolome and microbiome appear to be of particular importance in the aetiology of type 2 diabetes.
The microbiome
The gut microbiota is known for its ability to modulate inflammation, metabolise xenobiotics, maintain intestinal integrity and its interactions with dietary components. Changes in the abundance and diversity of the gut microbiota have been linked to the progression of many metabolic diseases, including type 2 diabetes [81,82,83]. The specific associations between the gut microbiome and type 2 diabetes in humans have conveyed conflicting results, which can be partly explained by large methodological differences in microbiome studies [81,82,83]. Nevertheless, an analysis of over 40 observational studies has identified specific members of the gut microbiome that appear to be consistently associated with type 2 diabetes [81]. Five genera showed a recurrent protective role in relation to type 2 diabetes (Bacteroides, Bifidobacterium, Akkermansia, Roseburia and Faecalibacterium). These genera are associated with several metabolic mechanisms that affect host physiology, such as reduction of endotoxemia, increase of energy yield, drug metabolism, decrease of tissue inflammation and production of bacterial metabolites [81, 82, 84,85,86]. Gou et al recently applied a machine learning framework to correlate gut microbiome features to type 2 diabetes in large scale cohorts [87]. They demonstrated that a microbial risk score of 14 microbial features (MRS) yielded a superior disease prediction accuracy than other environmental aspects (i.e. lifestyle, diet and host genetics). The combination of all factors, however, showed the highest predictive accuracy [87]. Nonetheless, this study highlights the role of the microbiome in type 2 diabetes and the complex interaction of the gut microbiome with environmental factors, and their link to the onset and progression of type 2 diabetes requires further research.
Metabolome and exposome scans
Technological developments, in which untargeted liquid- (LC-) and gas (GC-) chromatography are combined with high-resolution mass spectrometry (HRMS), have made it possible to comprehensively, and in a high-throughput fashion, measure the patterns of thousands of metabolites that are present in biological fluids, known as the metabolome [88, 89]. The metabolome provides a picture of the functional status of the biological system and as such can provide insights into the pathophysiology of type 2 diabetes and enable identification of type 2 diabetes biomarkers [88, 89]. Several endogenous metabolites were identified as early biomarkers of type 2 diabetes, including branched-chain amino acids, aromatic amino acids, 2-aminoadipic acid, sphingomyelin, glycine, acyl-alkyl-phosphatidylcholines, lysophosphatidylcholine, hexose, β-hydroxybutyrate, linoleoylglycerophosphocholine, and glyoxylate [89,90,91,92,93,94].
In light of the possibilities for future (public health) interventions, it is of interest to assess which environmental factors impact type 2 diabetes directly or through perturbations of the metabolome. In the USA, Patel et al measured 266 environmental factors in urine or blood as part of the National Health and Nutrition Examination Survey [95]. The pesticide derivative heptachlor epoxide, vitamin γ-tocopherol, and specific polychlorinated biphenyls were identified as risk factors for type 2 diabetes. β-Carotenes (among others) were identified as protective factors for type 2 diabetes.
Recent optimisation of LC-HRMS and GC-HRMS platforms combined with innovative data extraction approaches now enable the detection of an even wider range of exposure-related chemicals present at very low concentrations in biological fluids (e.g. blood and urine) [15]. Application of these technologies to human populations allows detection and characterisation of a large range of exogenously derived small chemicals, including pharmaceuticals, pesticides, preservatives, dietary compounds and microbial metabolites, in small quantities of biological materials [96]. Collectively, these measurements provide a snapshot of the internal exposome, which can be used to elucidate some of the underlying biological mechanisms of known or suspected risk factors (such as heavy metals [97,98,99,100], other trace elements [101], persistent organic pollutants [102, 103], drug use [104] and air pollution [105]) on the development of type 2 diabetes.
Methodological considerations
Our living environment is mostly an indirect determinant of type 2 diabetes and changes in the living environment are often difficult to investigate in controlled studies. Therefore, the majority of evidence on environmental risk factors of type 2 diabetes stems from observational studies with relatively limited evidence from natural experiments. Causal inference based on observational studies is challenging, since they can be susceptible to reverse causation and confounding, as residence preference and selection is not a random process [106]. Although most studies adjust for relevant confounding factors like socioeconomic position, most studies cannot control for processes underlying choice of residence. Moreover, environmental characteristics often correlate with a certain location. For example, urbanisation is often associated with neighbourhood socioeconomic position, and certain food outlets also cluster in highly urbanised neighbourhoods with lower socioeconomic position. Therefore, conclusions on causality of the observed associations can only be drawn for environmental factors where natural experiments or well-controlled observational studies of residential relocation confirm associations found in observational studies. Based on the current evidence, this mainly holds true for neighbourhood deprivation, green space and walkability. For other environmental factors, longitudinal studies, and particularly natural experiments, are needed to confirm observed associations. Such studies should account for changes in the living environment in relation to incidence of type 2 diabetes either by selecting a cohort of people moving to a different location to study the changes in living environment or by selecting a cohort of people residing in the same location for a longer time period to study environmental changes in this neighbourhood in relation to type 2 diabetes. However, such studies thus far are scarce. Furthermore, for certain environmental factors, with the exception of the food environment [35], changes over time will be small, and development of type 2 diabetes is slow, warranting long follow-up durations [107, 108]. Natural experiments, for instance implementing a new car-free cycling zone or limiting fast-food outlets in certain regions, may offer additional evidence by creating a sudden and larger change in the environment. Furthermore, most studies have focused on the residential living environment, while activity space (routes, destinations and work environments) is likely to be relevant, as well as an individual’s interaction with their environments [109]. Finally, although urban residence is associated with an increased risk of type 2 diabetes compared with rural residence, particularly in low- and middle-income countries [26], for other environmental risk factors most evidence comes from high-income countries [20, 25, 26]. With the exception of a few studies addressing effect modification by sex, ethnicity, income or other characteristics [28, 110], little is known about differential effects of environmental factors across subpopulations based on sex or ethnicity, which should be further investigated.
A more thorough understanding of the underlying biological pathways linking the environment to risk of type 2 diabetes may also help in assessing causality. In addition to providing deeper biological insights into previously identified risk factors, assessment of the internal exposome will contribute to the identification of currently unknown risk factors of type 2 diabetes, and will provide insight into how these exogenous chemicals collectively interact with type 2 diabetes risk profiles [11]. Such insights require the application of advanced methods for annotation [96], and statistical and biological interpretation of the generated data [11]. Large studies are needed to make solid inferences using complex and high-dimensional data.
Even though high-quality methods are available today, progress can still be made in terms of the quality of the assessment of the living environment. This pertains to both the external and the internal exposome. The type of tools and methods that are needed varies from domain to domain but should typically incorporate the ability to assess a wide range of factors with high sensitivity. A recent review provided a critical overview of the tools and methods that are currently available for exposome studies and indicated where progress can still be made [15].
Future perspectives
Future studies should investigate longitudinal changes in our environment in relation to risk of type 2 diabetes, including pathway analyses of health behaviours. Effects of our environment on the microbiome or endogenous metabolites should also be incorporated to investigate underlying pathways in the association of our environment with type 2 diabetes through effects on stress responses or inflammation. By analysing not only the gut microbiome in relation to the disease, but also considering the influence of other environmental exposures, such as diet, pollution and medication, as well as host metabolism, we expect to be able to better understand the role of the gut microbiome in type 2 diabetes. For example, for light at night, more research is necessary to understand the underlying mechanism driving the association with increased risk of type 2 diabetes, as it is suggested that hormone levels, circadian rhythms or sleep quality, may play a role in these associations [48].
For the food environment, exposure should be operationalised more accurately. More insight is needed into mobility patterns combined with behavioural insights on use of food retailers and food delivery services, and perceptions of the food environment. This can be done by detailed assessment of these environmental exposures over time in smaller longitudinal observational studies that account for exposures other than the residential environment and activity space of individuals. These studies can contribute to a more accurate exposure assessment in cohort studies or registries to investigate the association with incidence of type 2 diabetes. The exposure assessment in cohorts could be improved using regression calibration techniques when the necessary data are available. Otherwise, assessment should be improved by incorporating relevant exposure measures in follow-up questionnaires. For the social environment, future research should account for methodological challenges in investigating the link between social environment and type 2 diabetes risk, such as inconsistent conceptualisations of the social environment, measurements unable to tease apart different social phenomena operating at multiple levels of influence, long time lag between exposure and impact on type 2 diabetes development, and risk accumulation over the life course [58, 72, 111].
For green space, walkability, air pollution and neighbourhood socioeconomic position, consistent and robust associations with type 2 diabetes have been documented. For these aspects, structural interventions should be evaluated for their effect on risk of type 2 diabetes and other chronic diseases using health impact modelling or natural experiments. These studies will contribute to urban planning and help shape a healthier living environment.
Conclusion
In conclusion, our environment accounts for a substantial proportion of disease burden due to type 2 diabetes. Studies have provided consistent evidence that air pollution, residential noise, neighbourhood walkability, green space and area-level socioeconomic deprivation are associated with risk of type 2 diabetes. Current evidence for the contribution of the food environment, other aspects of the social environment and temperature is low or inconsistent. Our environment is thought to affect the risk of type 2 diabetes mainly through mechanisms incorporating lifestyle factors such as physical activity or diet, the microbiome, inflammation or chronic stress. Future studies should focus on investigating the longitudinal association of changes in our environment in relation to risk of type 2 diabetes and whether these associations are explained by these proposed mechanisms. When robust evidence for the association of environmental factors with risk of type 2 diabetes is available, natural experiments or health impact modelling can help to evaluate the impact of environmental interventions on disease burden. This will contribute to urban planning and help shape a healthier living environment.
Abbreviations
- HRMS:
-
High-resolution mass spectrometry
References
Standl E, Khunti K, Hansen TB, Schnell O (2019) The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol 26(2_suppl):7–14. https://doi.org/10.1177/2047487319881021
Dal Canto E, Ceriello A, Ryden L et al (2019) Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol 26(2_suppl):25–32. https://doi.org/10.1177/2047487319878371
Uusitupa M, Khan TA, Viguiliouk E et al (2019) Prevention of type 2 diabetes by lifestyle changes: a systematic review and Meta-analysis. Nutrients 11(11). https://doi.org/10.3390/nu11112611
Duijzer G, Bukman AJ, Meints-Groenveld A et al (2019) Cost-effectiveness of the SLIMMER diabetes prevention intervention in Dutch primary health care: economic evaluation from a randomised controlled trial. BMC Health Serv Res 19(1):824. https://doi.org/10.1186/s12913-019-4529-8
Yoon U, Kwok LL, Magkidis A (2013) Efficacy of lifestyle interventions in reducing diabetes incidence in patients with impaired glucose tolerance: a systematic review of randomized controlled trials. Metabolism 62(2):303–314. https://doi.org/10.1016/j.metabol.2012.07.009
Backholer K, Beauchamp A, Ball K et al (2014) A framework for evaluating the impact of obesity prevention strategies on socioeconomic inequalities in weight. Am J Public Health 104(10):e43–e50. https://doi.org/10.2105/AJPH.2014.302066
Mahajan A, Taliun D, Thurner M et al (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50(11):1505–1513. https://doi.org/10.1038/s41588-018-0241-6
Fuchsberger C, Flannick J, Teslovich TM et al (2016) The genetic architecture of type 2 diabetes. Nature 536(7614):41–47. https://doi.org/10.1038/nature18642
Li Y, Wang DD, Ley SH et al (2017) Time trends of dietary and lifestyle factors and their potential impact on diabetes burden in China. Diabetes Care 40(12):1685–1694. https://doi.org/10.2337/dc17-0571
Wild CP (2005) Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark Prev 14(8):1847–1850. https://doi.org/10.1158/1055-9965.EPI-05-0456
Vermeulen R, Schymanski EL, Barabasi AL, Miller GW (2020) The exposome and health: where chemistry meets biology. Science 367(6476):392–396. https://doi.org/10.1126/science.aay3164
Pourchet M, Debrauwer L, Klanova J et al (2020) Suspect and non-targeted screening of chemicals of emerging concern for human biomonitoring, environmental health studies and support to risk assessment: from promises to challenges and harmonisation issues. Environ Int 139:105545. https://doi.org/10.1016/j.envint.2020.105545
Guida F, Sandanger TM, Castagne R et al (2015) Dynamics of smoking-induced genome-wide methylation changes with time since smoking cessation. Hum Mol Genet 24(8):2349–2359. https://doi.org/10.1093/hmg/ddu751
Chadeau-Hyam M, Bodinier B, Vermeulen R et al (2020) Education, biological ageing, all-cause and cause-specific mortality and morbidity: UK biobank cohort study. EClinicalMedicine 29-30:100658. https://doi.org/10.1016/j.eclinm.2020.100658
Niedzwiecki MM, Walker DI, Vermeulen R, Chadeau-Hyam M, Jones DP, Miller GW (2019) The Exposome: molecules to populations. Annu Rev Pharmacol Toxicol 59:107–127. https://doi.org/10.1146/annurev-pharmtox-010818-021315
Bellou V, Belbasis L, Tzoulaki I, Evangelou E (2018) Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One 13(3):e0194127. https://doi.org/10.1371/journal.pone.0194127
Glanz K, Sallis JF, Saelens BE, Frank LD (2005) Healthy nutrition environments: concepts and measures. Am J Health Promot 19(5):330–333, ii. https://doi.org/10.4278/0890-1171-19.5.330
Walker RE, Keane CR, Burke JG (2010) Disparities and access to healthy food in the United States: a review of food deserts literature. Health Place 16(5):876–884. https://doi.org/10.1016/j.healthplace.2010.04.013
Caspi CE, Sorensen G, Subramanian SV, Kawachi I (2012) The local food environment and diet: a systematic review. Health Place 18(5):1172–1187. https://doi.org/10.1016/j.healthplace.2012.05.006
Dendup T, Feng X, Clingan S, Astell-Burt T (2018) Environmental risk factors for developing type 2 diabetes mellitus: a systematic review. Int J Environ Res Public Health 15(1). https://doi.org/10.3390/ijerph15010078
Lytle LA, Sokol RL (2017) Measures of the food environment: a systematic review of the field, 2007-2015. Health Place 44:18–34. https://doi.org/10.1016/j.healthplace.2016.12.007
Boone-Heinonen J, Gordon-Larsen P, Kiefe CI, Shikany JM, Lewis CE, Popkin BM (2011) Fast food restaurants and food stores: longitudinal associations with diet in young to middle-aged adults: the CARDIA study. Arch Intern Med 171(13):1162–1170. https://doi.org/10.1001/archinternmed.2011.283
Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR Jr, Popkin BM (2009) Regular consumption from fast food establishments relative to other restaurants is differentially associated with metabolic outcomes in young adults. J Nutr 139(11):2113–2118. https://doi.org/10.3945/jn.109.109520
Pereira MA, Kartashov AI, Ebbeling CB et al (2005) Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet 365(9453):36–42. https://doi.org/10.1016/S0140-6736(04)17663-0
Bilal U, Auchincloss AH, Diez-Roux AV (2018) Neighborhood environments and diabetes risk and control. Curr Diab Rep 18(9):62. https://doi.org/10.1007/s11892-018-1032-2
den Braver NR, Lakerveld J, Rutters F, Schoonmade LJ, Brug J, Beulens JWJ (2018) Built environmental characteristics and diabetes: a systematic review and meta-analysis. BMC Med 16(1):12. https://doi.org/10.1186/s12916-017-0997-z
Auchincloss AH, Diez Roux AV, Mujahid MS, Shen M, Bertoni AG, Carnethon MR (2009) Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis. Arch Intern Med 169(18):1698–1704. https://doi.org/10.1001/archinternmed.2009.302
Christine PJ, Auchincloss AH, Bertoni AG et al (2015) Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis (MESA). JAMA Intern Med 175(8):1311–1320. https://doi.org/10.1001/jamainternmed.2015.2691
Hikichi H, Aida J, Kondo K, Tsuboya T, Kawachi I (2019) Residential relocation and obesity after a natural disaster: a natural experiment from the 2011 Japan earthquake and tsunami. Sci Rep 9(1):374. https://doi.org/10.1038/s41598-018-36906-y
Shiba K, Hanazato M, Aida J et al (2020) Cardiometabolic profiles and change in neighborhood food and built environment among older adults: a natural experiment. Epidemiology 31(6):758–767. https://doi.org/10.1097/EDE.0000000000001243
Frankenfeld CL, Leslie TF, Makara MA (2015) Diabetes, obesity, and recommended fruit and vegetable consumption in relation to food environment sub-types: a cross-sectional analysis of behavioral risk factor surveillance system, United States census, and food establishment data. BMC Public Health 15:491. https://doi.org/10.1186/s12889-015-1819-x
Mezuk B, Li X, Cederin K, Rice K, Sundquist J, Sundquist K (2016) Beyond access: characteristics of the food environment and risk of diabetes. Am J Epidemiol 183(12):1129–1137. https://doi.org/10.1093/aje/kwv318
James P, Seward MW, James O'Malley A, Subramanian SV, Block JP (2017) Changes in the food environment over time: examining 40 years of data in the Framingham heart study. Int J Behav Nutr Phys Act 14(1):84. https://doi.org/10.1186/s12966-017-0537-4
Maguire ER, Burgoine T, Monsivais P (2015) Area deprivation and the food environment over time: a repeated cross-sectional study on takeaway outlet density and supermarket presence in Norfolk, UK, 1990-2008. Health Place 33:142–147. https://doi.org/10.1016/j.healthplace.2015.02.012
Pinho MGM, Mackenbach JD, den Braver NR, Beulens JJW, Brug J, Lakerveld J (2020) Recent changes in the Dutch foodscape: socioeconomic and urban-rural differences. Int J Behav Nutr Phys Act 17(1):43. https://doi.org/10.1186/s12966-020-00944-5
Richardson AS, Meyer KA, Howard AG et al (2014) Neighborhood socioeconomic status and food environment: a 20-year longitudinal latent class analysis among CARDIA participants. Health Place 30:145–153. https://doi.org/10.1016/j.healthplace.2014.08.011
Perez-Ferrer C, Auchincloss AH, Barrientos-Gutierrez T et al (2020) Longitudinal changes in the retail food environment in Mexico and their association with diabetes. Health Place 66:102461. https://doi.org/10.1016/j.healthplace.2020.102461
Amuda AT, Berkowitz SA (2019) Diabetes and the built environment: evidence and policies. Curr Diab Rep 19(7):35. https://doi.org/10.1007/s11892-019-1162-1
Sallis JF, Floyd MF, Rodriguez DA, Saelens BE (2012) Role of built environments in physical activity, obesity, and cardiovascular disease. Circulation 125(5):729–737. https://doi.org/10.1161/CIRCULATIONAHA.110.969022
Gorman S, Larcombe AN, Christian HE (2021) Exposomes and metabolic health through a physical activity lens: a narrative review. J Endocrinol 249(1):R25–R41. https://doi.org/10.1530/JOE-20-0487
Smith M, Hosking J, Woodward A et al (2017) Systematic literature review of built environment effects on physical activity and active transport - an update and new findings on health equity. Int J Behav Nutr Phys Act 14(1):158. https://doi.org/10.1186/s12966-017-0613-9
Kivimaki M, Batty GD, Pentti J et al (2021) Modifications to residential neighbourhood characteristics and risk of 79 common health conditions: a prospective cohort study. Lancet Public Health 6(6):e396–e407. https://doi.org/10.1016/S2468-2667(21)00066-9
den Braver NR, Kok JG, Mackenbach JD et al (2020) Neighbourhood drivability: environmental and individual characteristics associated with car use across Europe. Int J Behav Nutr Phys Act 17(1):8. https://doi.org/10.1186/s12966-019-0906-2
Fouladi F, Bailey MJ, Patterson WB et al (2020) Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ Int 138:105604. https://doi.org/10.1016/j.envint.2020.105604
Yang BY, Fan S, Thiering E et al (2020) Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res 180:108817. https://doi.org/10.1016/j.envres.2019.108817
Shan A, Zhang Y, Zhang LW et al (2020) Associations between the incidence and mortality rates of type 2 diabetes mellitus and long-term exposure to ambient air pollution: a 12-year cohort study in northern China. Environ Res 186:109551. https://doi.org/10.1016/j.envres.2020.109551
Afroz-Hossain A, Dawkins M, Myers AK (2019) Sleep and environmental factors affecting glycemic control in people with type 2 diabetes mellitus. Curr Diab Rep 19(7):40. https://doi.org/10.1007/s11892-019-1159-9
Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N (2014) Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol Int 31(3):394–400. https://doi.org/10.3109/07420528.2013.864299
Tan X, Chapman CD, Cedernaes J, Benedict C (2018) Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev 40:127–134. https://doi.org/10.1016/j.smrv.2017.11.001
Dzhambov AM (2015) Long-term noise exposure and the risk for type 2 diabetes: a meta-analysis. Noise Health 17(74):23–33. https://doi.org/10.4103/1463-1741.149571
Ouellet V, Routhier-Labadie A, Bellemare W et al (2011) Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96(1):192–199. https://doi.org/10.1210/jc.2010-0989
Hanssen MJ, Hoeks J, Brans B et al (2015) Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21(8):863–865. https://doi.org/10.1038/nm.3891
Ouellet V, Labbe SM, Blondin DP et al (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122(2):545–552. https://doi.org/10.1172/JCI60433
Blauw LL, Aziz NA, Tannemaat MR et al (2017) Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res Care 5(1):e000317. https://doi.org/10.1136/bmjdrc-2016-000317
Wu H, Bertrand KA, Choi AL et al (2013) Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses' health study and meta-analysis. Environ Health Perspect 121(2):153–161. https://doi.org/10.1289/ehp.1205248
Evangelou E, Ntritsos G, Chondrogiorgi M et al (2016) Exposure to pesticides and diabetes: a systematic review and meta-analysis. Environ Int 91:60–68. https://doi.org/10.1016/j.envint.2016.02.013
Misra BB, Misra A (2020) The chemical exposome of type 2 diabetes mellitus: opportunities and challenges in the omics era. Diabetes Metab Syndr 14(1):23–38. https://doi.org/10.1016/j.dsx.2019.12.001
Barnett E, Casper M (2001) A definition of "social environment". Am J Public Health 91(3):465–465. https://doi.org/10.2105/AJPH.91.3.465a
Kivimäki M, Virtanen M, Kawachi I et al (2015) Long working hours, socioeconomic status, and the risk of incident type 2 diabetes: a meta-analysis of published and unpublished data from 222120 individuals. Lancet Diabetes Endocrinol 3(1):27–34. https://doi.org/10.1016/S2213-8587(14)70178-0
Seiglie JA, Marcus ME, Ebert C et al (2020) Diabetes prevalence and its relationship with education, wealth, and BMI in 29 low- and middle-income countries. Diabetes Care 43(4):767–775. https://doi.org/10.2337/dc19-1782
Hill-Briggs F, Adler NE, Berkowitz SA et al (2020) Social determinants of health and Diabetes: A Scientific Review. Diabetes Care. https://doi.org/10.2337/dci20-0053
Müller G, Kluttig A, Greiser KH et al (2013) Regional and neighborhood disparities in the odds of type 2 diabetes: results from 5 population-based studies in Germany (DIAB-CORE consortium). Am J Epidemiol 178(2):221–230. https://doi.org/10.1093/aje/kws466
Ludwig J, Sanbonmatsu L, Gennetian L et al (2011) Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med 365(16):1509–1519. https://doi.org/10.1056/NEJMsa1103216
White JS, Hamad R, Li X et al (2016) Long-term effects of neighbourhood deprivation on diabetes risk: quasi-experimental evidence from a refugee dispersal policy in Sweden. Lancet Diabetes Endocrinol 4(6):517–524. https://doi.org/10.1016/S2213-8587(16)30009-2
Rodgers J, Valuev AV, Hswen Y, Subramanian SV (2019) Social capital and physical health: an updated review of the literature for 2007-2018. Soc Sci Med 236:112360. https://doi.org/10.1016/j.socscimed.2019.112360
Hu F, Hu B, Chen R et al (2014) A systematic review of social capital and chronic non-communicable diseases. Biosci Trends 8(6):290–296. https://doi.org/10.5582/bst.2014.01138
Riumallo-Herl CJ, Kawachi I, Avendano M (2014) Social capital, mental health and biomarkers in Chile: assessing the effects of social capital in a middle-income country. Soc Sci Med 105:47–58. https://doi.org/10.1016/j.socscimed.2013.12.018
Holtgrave DR, Crosby R (2006) Is social capital a protective factor against obesity and diabetes? Findings from an exploratory study. Ann Epidemiol 16(5):406–408. https://doi.org/10.1016/j.annepidem.2005.04.017
Schram MT, Assendelft WJJ, van Tilburg TG, Dukers-Muijrers N (2021) Social networks and type 2 diabetes: a narrative review. Diabetologia 64(9):1905–1916. https://doi.org/10.1007/s00125-021-05496-2
Kershaw KN, Pender AE (2016) Racial/ethnic residential segregation, obesity, and diabetes mellitus. Curr Diab Rep 16(11):108. https://doi.org/10.1007/s11892-016-0800-0
Whitaker KM, Everson-Rose SA, Pankow JS et al (2017) Experiences of discrimination and incident type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis (MESA). Am J Epidemiol 186(4):445–455. https://doi.org/10.1093/aje/kwx047
Dendup T, Astell-Burt T, Feng XQ (2019) Residential self-selection, perceived built environment and type 2 diabetes incidence: a longitudinal analysis of 36,224 middle to older age adults. Health Place 58. https://doi.org/10.1016/j.healthplace.2019.102154
Dendup T, Feng X, O'Shaughnessy PY, Astell-Burt T (2021) Role of perceived neighbourhood crime in the longitudinal association between perceived built environment and type 2 diabetes mellitus: a moderated mediation analysis. J Epidemiol Community Health 75(2):120–127. https://doi.org/10.1136/jech-2020-214175
Christakis NA, Fowler JH (2007) The spread of obesity in a large social network over 32 years. N Engl J Med 357(4):370–379. https://doi.org/10.1056/NEJMsa066082
Choi YJ, Ailshire JA, Crimmins EM (2020) Living alone, social networks in neighbourhoods, and daily fruit and vegetable consumption among middle-aged and older adults in the USA. Public Health Nutr 23(18):3315–3323. https://doi.org/10.1017/S1368980020002475
Shareck M, Ellaway A (2011) Neighbourhood crime and smoking: the role of objective and perceived crime measures. BMC Public Health 11:930. https://doi.org/10.1186/1471-2458-11-930
McNeill LH, Kreuter MW, Subramanian SV (2006) Social environment and physical activity: a review of concepts and evidence. Soc Sci Med 63(4):1011–1022. https://doi.org/10.1016/j.socscimed.2006.03.012
Ng Fat L, Scholes S, Jivraj S (2017) The relationship between drinking pattern, social capital, and area-deprivation: findings from the health survey for England. J Stud Alcohol Drugs 78(1):20–29. https://doi.org/10.15288/jsad.2017.78.20
Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A (2011) Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 40(3):804–818. https://doi.org/10.1093/ije/dyr029
Stringhini S, Batty GD, Bovet P et al (2013) Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the Whitehall II prospective cohort study. PLoS Med 10(7):e1001479. https://doi.org/10.1371/journal.pmed.1001479
Gurung M, Li Z, You H et al (2020) Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590. https://doi.org/10.1016/j.ebiom.2019.11.051
Sharma S, Tripathi P (2019) Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem 63:101–108. https://doi.org/10.1016/j.jnutbio.2018.10.003
Xu WT, Nie YZ, Yang Z, Lu NH (2016) The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiol 11:825–836. https://doi.org/10.2217/fmb-2015-0024
Canfora EE, Meex RCR, Venema K, Blaak EE (2019) Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol 15(5):261–273. https://doi.org/10.1038/s41574-019-0156-z
Li R, Andreu-Sanchez S, Kuipers F, Fu J (2021) Gut microbiome and bile acids in obesity-related diseases. Best Pract Res Clin Endocrinol Metab 101493. https://doi.org/10.1016/j.beem.2021.101493
Qin J, Li Y, Cai Z et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490(7418):55–60. https://doi.org/10.1038/nature11450
Gou W, Ling CW, He Y et al (2021) Interpretable machine learning framework reveals robust gut microbiome features associated with type 2 diabetes. Diabetes Care 44(2):358–366. https://doi.org/10.2337/dc20-1536
Gonzalez-Franquesa A, Burkart AM, Isganaitis E, Patti ME (2016) What have metabolomics approaches taught us about type 2 diabetes? Curr Diab Rep 16(8):74. https://doi.org/10.1007/s11892-016-0763-1
Wang TJ, Larson MG, Vasan RS et al (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17(4):448–453. https://doi.org/10.1038/nm.2307
Ferrannini E, Natali A, Camastra S et al (2013) Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 62(5):1730–1737. https://doi.org/10.2337/db12-0707
Floegel A, Stefan N, Yu Z et al (2013) Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62(2):639–648. https://doi.org/10.2337/db12-0495
Padberg I, Peter E, Gonzalez-Maldonado S et al (2014) A new metabolomic signature in type-2 diabetes mellitus and its pathophysiology. PLoS One 9(1):e85082. https://doi.org/10.1371/journal.pone.0085082
Wang TJ, Ngo D, Psychogios N et al (2013) 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 123(10):4309–4317. https://doi.org/10.1172/JCI64801
Wang-Sattler R, Yu Z, Herder C et al (2012) Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 8:615. https://doi.org/10.1038/msb.2012.43
Patel CJ, Bhattacharya J, Butte AJ (2010) An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One 5(5):e10746. https://doi.org/10.1371/journal.pone.0010746
Meijer J, Lamoree M, Hamers T et al (2021) An annotation database for chemicals of emerging concern in exposome research. Environ Int 152:106511. https://doi.org/10.1016/j.envint.2021.106511
Barregard L, Bergstrom G, Fagerberg B (2013) Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environ Res 121:104–109. https://doi.org/10.1016/j.envres.2012.11.005
He K, Xun P, Liu K, Morris S, Reis J, Guallar E (2013) Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA trace element study. Diabetes Care 36(6):1584–1589. https://doi.org/10.2337/dc12-1842
Hendryx M, Luo J, Chojenta C, Byles JE (2019) Exposure to heavy metals from point pollution sources and risk of incident type 2 diabetes among women: a prospective cohort analysis. Int J Environ Health Res 1–12. https://doi.org/10.1080/09603123.2019.1668545
Lin JL, Lin-Tan DT, Yu CC, Li YJ, Huang YY, Li KL (2006) Environmental exposure to lead and progressive diabetic nephropathy in patients with type II diabetes. Kidney Int 69(11):2049–2056. https://doi.org/10.1038/sj.ki.5001505
Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E (2008) Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300(7):814–822. https://doi.org/10.1001/jama.300.7.814
Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H (2011) Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care 34(9):1972–1979. https://doi.org/10.2337/dc10-2303
Wolf K, Bongaerts BWC, Schneider A et al (2019) Persistent organic pollutants and the incidence of type 2 diabetes in the CARLA and KORA cohort studies. Environ Int 129:221–228. https://doi.org/10.1016/j.envint.2019.05.030
Boursi B, Mamtani R, Haynes K, Yang YX (2015) The effect of past antibiotic exposure on diabetes risk. Eur J Endocrinol 172(6):639–648. https://doi.org/10.1530/EJE-14-1163
Hansen AB, Ravnskjaer L, Loft S et al (2016) Long-term exposure to fine particulate matter and incidence of diabetes in the Danish nurse cohort. Environ Int 91:243–250. https://doi.org/10.1016/j.envint.2016.02.036
Cao XY, Mokhtarian PL, Handy SL (2009) Examining the impacts of residential self-selection on travel behaviour: a focus on empirical findings. Transp Rev 29(3):359–395. https://doi.org/10.1080/01441640802539195
McCormack GR, Shiell A (2011) In search of causality: a systematic review of the relationship between the built environment and physical activity among adults. Int J Behav Nutr Phys Act 8:Artn 125. https://doi.org/10.1186/1479-5868-8-125
Drewnowski A, Buszkiewicz J, Aggarwal A, Rose C, Gupta S, Bradshaw A (2020) Obesity and the built environment: a reappraisal. Obesity (Silver Spring) 28(1):22–30. https://doi.org/10.1002/oby.22672
Lin CY, Koohsari MJ, Liao Y et al (2020) Workplace neighbourhood built environment and workers' physically-active and sedentary behaviour: a systematic review of observational studies. Int J Behav Nutr Phys Act 17(1):ARTN 148. https://doi.org/10.1186/s12966-020-01055-x
Cunningham-Myrie CA, Theall KP, Younger NO et al (2015) Associations between neighborhood effects and physical activity, obesity, and diabetes: the Jamaica health and lifestyle survey 2008. J Clin Epidemiol 68(9):970–978. https://doi.org/10.1016/j.jclinepi.2014.08.004
Diez Roux AV, Mair C (2010) Neighborhoods and health. Ann N Y Acad Sci 1186:125–145. https://doi.org/10.1111/j.1749-6632.2009.05333.x
Wild CP (2012) The exposome: from concept to utility. Int J Epidemiol 41(1):24–32. https://doi.org/10.1093/ije/dyr236
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. JWJB declares to be a member of the editorial board of Diabetologia.
Funding
Work in the authors’ laboratories is supported by EXPOSOME-NL and EXPANSE. EXPOSOME-NL is funded through the Gravitation program of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant number 024.004.017). EXPANSE has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 874627.
Author information
Authors and Affiliations
Contributions
All authors were responsible for drafting the article and revising it critically for important intellectual content. All authors approved the version to be published.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PPTX 536 kb)
Rights and permissions
About this article
Cite this article
Beulens, J.W.J., Pinho, M.G.M., Abreu, T.C. et al. Environmental risk factors of type 2 diabetes—an exposome approach. Diabetologia 65, 263–274 (2022). https://doi.org/10.1007/s00125-021-05618-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05618-w