Abstract
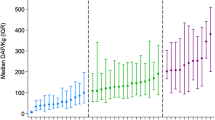
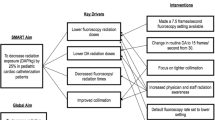
The objective of this study was to evaluate the impact of the regular introduction of new technologies into interventional cardiac catheterization procedures, in this case new atrial septal defect (ASD) closure devices, while conducting a multi-center collaborative initiative to reduce radiation usage during all procedures. Data were collected prospectively by 8 C3PO institutions between January 1, 2014 and December 31, 2017 for ASD device closure procedures in the cardiac catheterization lab during a quality improvement (QI) initiative aimed at reducing patient radiation exposure. Radiation exposure was measured in dose area product per body weight (µGy*m2/kg). Use of proposed practice change strategies at the beginning and end of the QI intervention period was assessed. Radiation exposure was summarized by institution and by initial type of device used for closure. This study included 602 ASD device closures. Without changes in patient characteristics, total fluoroscopy duration, or number of digital acquisitions, median radiation exposure decreased from 37 DAP/kg to 14 DAP/kg from 2014 to 2017. While all individual centers decreased overall median DAP/kg, the use of novel devices for ASD closure correlated with a temporary period of worsening institutional radiation exposure and increased fluoroscopy time. The introduction of new ASD closure devices resulted in increased radiation exposure during a QI project designed to reduce radiation exposure. Therefore, outcome assessment must be contextualized in QI projects, hospital evaluation, and public reporting, to acknowledge the expected variation during innovation and introduction of novel therapies.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, LB, upon reasonable request.
Abbreviations
- ASD:
-
Atrial septal defect
- DAP:
-
Dose area product
- QI:
-
Quality improvement
- C3PO:
-
Congenital cardiac catheterization project on outcomes (C3PO)
- PVR:
-
Pulmonary vascular resistance
- AE:
-
Adverse event
- GSO:
-
GORE® CARDIOFORM Septal Occluder
References
Zampi JD, Whiteside W (2018) Innovative interventional catheterization techniques for congenital heart disease. Transl Pediatr 7(2):104–119
Kang S-L, Benson L (2018) Recent advances in cardiac catheterization for congenital heart disease. F1000Research 26(7):370
Huang RL, Donelli A, Byrd J, Mickiewicz MA, Slovis C, Roumie C et al (2008) Using quality improvement methods to improve door-to-balloon time at an academic medical center. J Invasive Cardiol 20(2):46–52
Frey P, Connors A, Resnic FS (2012) Quality measurement and improvement in the cardiac catheterization laboratory. Circulation 125(4):615–619
Dehmer GJ, Hirshfeld JW, Oetgen WJ, Mitchell K, Simon AW, Elma M et al (2004) CathKIT: improving quality in the cardiac catheterization laboratory. J Am Coll Cardiol 43(5):893–899
Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP (2009). In: Langley GJ (ed) The improvement guide a practical approach to enhancing organizational performance, 2nd edn. Jossey-Bass, San Francisco
Uretsky BF, Wang FW (2006) Implementation and application of a continuous quality improvement (CQI) program for the cardiac catheterization laboratory: one institution’s 10-year experience. Catheter Cardiovasc Interv 68(4):586–595
Quinn BP, Armstrong AK, Bauser-Heaton HD, Callahan R, El-Said HG, Foerster SR et al (2019) Radiation risk categories in cardiac catheterization for congenital heart disease: a tool to aid in the evaluation of radiation outcomes. Pediatr Cardiol 40(2):445–453
Quinn BP, Cevallos P, Armstrong A, Balzer D, El-Said H, Foerster S et al (2020) Longitudinal improvements in radiation exposure in cardiac catheterization for congenital heart disease: a prospective multicenter C3PO-QI study. Circ Cardiovasc Interv. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008172
Cevallos PC, Rose MJ, Armsby LB, Armstrong AK, El-Said H, Foerster SR et al (2016) Implementation of methodology for quality improvement in pediatric cardiac catheterization: a multi-center initiative by the congenital cardiac catheterization project on outcomes-quality improvement (C3PO-QI). Pediatr Cardiol 37(8):1436–1445
Cevallos PC, Armstrong AK, Glatz AC, Goldstein BH, Gudausky TM, Leahy RA et al (2017) Radiation dose benchmarks in pediatric cardiac catheterization: a prospective multi-center C3PO-QI study. Catheter Cardiovasc Interv 90(2):269–280
Nykanen DG, Forbes TJ, Du W, Divekar AA, Reeves JH, Hagler DJ et al (2016) CRISP: catheterization RISk score for pediatrics: a report from the congenital cardiac interventional study consortium (CCISC). Catheter Cardiovasc Interv 87(2):302–309
Bergersen L, Gauvreau K, Foerster SR, Marshall AC, McElhinney DB, Beekman RH 3rd et al (2011) Catheterization for congenital heart disease adjustment for risk method (CHARM). JACC Cardiovasc Interv 4(9):1037–1046
Vincent RN, Moore J, Beekman RH, Benson L, Bergersen L, Holzer R et al (2016) Procedural characteristics and adverse events in diagnostic and interventional catheterisations in paediatric and adult CHD: initial report from the IMPACT Registry. Cardiol Young 26(1):70–78
El-Said HG, Hegde S, Foerster S, Hellenbrand W, Kreutzer J, Trucco SM et al (2015) Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.25684
Bergersen L, Gauvreau K, Marshall A, Kreutzer J, Beekman R, Hirsch R et al (2011) Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circ Cardiovasc Interv 4(2):188–194
Bergersen L, Marshall A, Gauvreau K, Beekman R, Hirsch R, Foerster S et al (2010) Adverse event rates in congenital cardiac catheterization—a multi-center experience. Catheter Cardiovasc Interv 75(3):389–400
Bergersen L, Gauvreau K, Jenkins KJ, Lock JE (2008) Adverse event rates in congenital cardiac catheterization: a new understanding of risks. Congenit Heart Dis 3(2):90–105
Bergersen L, Giroud JM, Jacobs JP, Franklin RCG, Béland MJ, Krogmann ON et al (2011) Report from the international society for nomenclature of paediatric and congenital heart disease: cardiovascular catheterisation for congenital and paediatric cardiac disease (part 2—nomenclature of complications associated with interventional cardiology. Cardiol Young 21(3):260–265
Gillespie MJ, Javois AJ, Moore P, Forbes T, Paolillo JA (2020) Use of the GORE® CARDIOFORM septal occluder for percutaneous closure of secundum atrial septal defects: Results of the multicenter U.S. IDE trial. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.28814
Sommer RJ, Love BA, Paolillo JA, Gray RG, Goldstein BH, Morgan GJ et al (2020) ASSURED clinical study: new GORE® CARDIOFORM ASD occluder for transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv 95(7):1285–1295
Quinn BP, Yeh M, Gauvreau K et al (2021) Procedural risk in congenital cardiac catheterization (PREDIC3T). J Am Heart Assoc. https://doi.org/10.1161/JAHA.121.022832
Hiremath G, Meadows J, Moore P (2015) How slow can we go? 4 frames per second (fps) versus 7.5 fps fluoroscopy for atrial septal defects (ASDs) device closure. Pediatr Cardiol. https://doi.org/10.1007/s00246-015-1122-8
Boudjemline Y (2018) Effects of reducing frame rate from 7.5 to 4 frames per second on radiation exposure in transcatheter atrial septal defect closure. Cardiol Young. https://doi.org/10.1017/S1047951118001257
Sitefane F, Malekzadeh-Milani S, Villemain O, Ladouceur M, Boudjemline Y (2018) Reduction of radiation exposure in transcatheter atrial septal defect closure: how low must we go? Arch Cardiovasc Dis. https://doi.org/10.1016/j.acvd.2017.05.011
Acknowledgements
None.
Funding
The research leading to these results received funding from the Children’s Heart Foundation (Northbrook, IL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no other relevant financial or non-financial relationships to disclose.
Ethics Approval
IRB approval for this study was obtained at Boston Children’s Hospital and was sought at participating institutions in accordance with local requirements.
Consent to Participate and Publication
The Boston Children’s IRB determined that this study met regulatory requirements necessary to waive the need for informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yeh, M.J., Shirley, L., Balzer, D.T. et al. Interpreting Quality Improvement When Introducing New Technology: A Collaborative Experience in ASD Device Closures. Pediatr Cardiol 43, 596–604 (2022). https://doi.org/10.1007/s00246-021-02762-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-021-02762-3