Abstract
Background
Accurate pre-operative diagnosis of orbital lesions supports appropriate prioritisation of patients into available theatre time. We examine the accuracy of pre-operative clinico-radiological diagnosis in a tertiary centre with weekly dedicated orbital clinics and associated multi-disciplinary team meetings.
Methods
A retrospective case notes review was undertaken for all patients who had an orbital biopsy performed at Bristol Eye Hospital between 2007 and 2017. In this centre, pre-operative clinico-radiological differential diagnoses are discussed during multi-disciplinary team meetings including two orbital specialist ophthalmologists and a specialist neuro-radiologist. Clinico-radiological diagnoses were compared with histopathological outcomes. Subcategory analysis according to histopathological diagnosis was undertaken to look for trends.
Results
172 biopsies were taken from 156 patients, median age 59 years (range 3 months to 91 years). 60.9% of patient were females, with equal numbers of right and left-sided biopsies. 11 patients had inconclusive histopathology. 15 patients did not have a documented preoperative diagnosis or differential offered in available notes. 71 patients (49.0%) demonstrated an exact match between clinico-radiological and histopathological diagnosis, 93 (64.1%) demonstrated a category match (e.g. inflammatory, lymphoproliferative) and for 111 (76.6%), the histopathological diagnosis was considered within the list of proffered clinico-radiological differential diagnoses.
Conclusions
Accuracy of pre-operative diagnosis of orbital lesions undergoing biopsy was higher in our series than previously reported by Koukoulli et al. Specialist head and neck radiology input via regular orbital multi-disciplinary meetings might be reciprocally educational and explain this difference. The authors recommend all surgeons who perform orbital surgery should have access to such multi-disciplinary meetings.
Similar content being viewed by others
Introduction
A wide variety of local and syste'mic diseases can present with masses in the orbit, and it is important for clinicians to plan surgeries and prioritise urgency of biopsies to confirm diagnosis and support appropriate treatment. Orbital imaging is routinely used as part of a pre-operative work-up to help support or refine the clinician’s assessment, and to aid surgical planning by confirming the size, location and characteristics of any lesion. Clinicians and radiologists are mutually dependent upon the quality of information provided to them when interpreting findings. Our tertiary unit benefits from input from a neuro-radiologist with orbital special interest at weekly multi-disciplinary team (MDT) meetings that occur immediately before the orbital clinic. Here, clinical information can be expanded upon, specific clinical questions posed, radiological features demonstrated, and initial treatment plans created. We reviewed a 10-year series of biopsies to determine the accuracy of our combined clinico-radiological (CR) diagnosis when compared with the gold standard of histopathological diagnosis and to compare this with levels previously reported in literature.
Materials and methods
All patients who underwent orbital biopsy between 2007 and 2017 in a single tertiary centre were identified via electronic medical record audit (Medisoft). Notes, histopathology, and radiological reports were retrospectively reviewed. Pre-operative diagnosis as documented after the orbital MDT (CR diagnosis) was compared with the ‘gold standard’ of histopathological diagnosis; where more than one differential diagnosis was offered, the first diagnosis documented was considered the top differential and used for analysis. Comparison was made between CR diagnosis and histopathology looking for an ‘exact match’ (e.g. both said sarcoidosis) or ‘category match’ (e.g. CR diagnosis was ‘inflammatory’ and histopathological diagnosis was ‘IgG4-related disease’); where neither of these matches occurred, the list of CR differentials offered were checked to see whether the correct diagnosis had been considered. The number of offered differential diagnoses considered was recorded. Where no differential diagnosis was offered this was classified as no match occurring. Categories used were as follows: inflammatory, lymphoproliferative, benign cystic, benign other, vascular, primary (nonlacrimal gland) malignancy, secondary (nonlacrimal gland) malignancy, isolated lacrimal gland tumour, peripheral nerve sheath tumour, optic nerve or meningeal tumour, and ‘normal’ (no identifiable pathology). Sensitivity and positive predictive values (PPV) were calculated for each category.
Results
172 biopsies were taken from 156 patients, with a median age of 59 years (range 3 months to 91 years). 60.9% of patients were females, and there was no preponderance for laterality, with equal number of biopsies from right and left orbits. 11 patients had inconclusive histopathology – these were excluded from analysis. 15 patients did not have a documented pre-operative diagnosis or differential offered. 71/145 patients (49%) demonstrated an exact match between CR and histopathological diagnosis, 93/145 (64.1%) demonstrated a category match (e.g. inflammatory, lymphoproliferative) and for 111/145 (76.6%), the histopathological diagnosis was considered within the proffered clinico-radiological differential. Table 1 gives a detailed breakdown of frequency of matches by category.
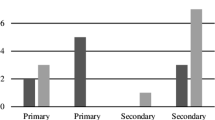
Inflammatory disorders occurred most commonly, affecting 40% of patients; lymphoproliferative disorders were next most common, affecting 27.6% of patients. Final diagnoses can be seen in Table 2. The highest CR diagnosis sensitivities occurred in normal tissue (100%), secondary malignancies (80%) and lacrimal gland tumours (75%). The CR diagnoses with the highest positive predictive values were normal tissue, lymphoproliferative disorders (74.4%) and benign, other lesions (66.7%). A single CR diagnosis was given for 44% of patients with a range of 0–6 diagnoses – see Fig. 1. Where no diagnosis was given, an anatomical description e.g. ‘orbital mass’ was usually documented instead. There was no clear correlation between the number of offered differentials and final diagnosis. Documentation of a diagnosis or differential diagnosis was offered in 81.7% of examined radiological reports – 76.9% of external reports and 82.3% of those reported within our trust, which was not statistically significant on Chi square testing.
10 patients required repeat biopsy during the study period, for which 50% affected the final diagnosis or management (e.g. non-specific inflammation later diagnosed as granulomatosis with polyangiitis, or lymphoma), and 1 patient still had inconclusive histopathology.
Discussion
Combined efforts of clinicians and radiologists
Clinicians and radiologists are mutually dependent on the information gleaned from each other for interpretation of findings. Supplying a succinct but comprehensive outline of the history, relevant clinical features and clinical question when requesting radiological imaging is important to help guide radiologists in deciding the most appropriate imaging protocol, and interpreting the images generated. Sometimes imaging may be requested by non-specialists, for example in ophthalmology casualty clinics, and the clinical question may not be clear, or their priority may be different from that of an orbital surgeon. MDTs with orbital and radiology specialists can deliver more clinical information, available from the patient records, and offers opportunity for more specialist queries to be answered. For example, a clinician in casualty clinic may be interested in confirming whether an orbital mass is present, where the orbital surgeon may wish to clarify the relative position or involvement of adjacent structures, which may impact their approach to biopsy. MDTs also provide opportunity for radiologists to highlight particular features on imaging that affect the likelihood of a particular diagnosis, building on previous intellectual knowledge and enhancing learning by creating links to real cases, which is rooted in cognitivism learning theory [1]. This has merits for the whole team, including residents doing subspecialty rotations, for whom such learning may prove invaluable in future roles where they may encounter orbital disease in a general ophthalmology/on-call role, or where access to specialist radiologists is challenging.
Furthermore, MDTs create opportunities for reciprocal learning by increasing radiologists’ understanding of orbital specialists’ clinical questions and applications of imaging findings, which may further enhance written reports in future. The MDT setting allows post-operative review and discussion of cases where ‘surprise’ pathology results do not correlate with prebiopsy diagnosis, promoting experiential learning for clinicians. The described interdependency of radiologists and orbital specialists irrespective of local MDTs means it may be inappropriate to evaluate their pre-operative diagnostic capabilities separately. The CR sensitivity identified in this series (49% across all patients) is higher than those found by
Koukkoulli et al., who report 35.7% clinical accuracy and 30.4% radiological accuracy [2]. Bacorn et al. highlighted the benefit of developing radiologists with an orbital specialist interest, citing agreement rates of 60.4% between specialist radiologists and histopathology compared with 43.8% for external/non-specialist radiologist agreement rates [3]. They argue that orbital specialists should personally review and interpret imaging to improve their own clinico-histopathological agreement rates, and report impressive overall agreement rates of 75.7%. However, their paper lacks clarity regarding their analytical approach, making direct comparison with their results difficult to interpret compared with the transparent processes outlined by Koukkoulli et al. and ourselves. Whilst we agree that surgeons should review images themselves, particularly before operating, Bacorn et al. suggest discussing imaging with radiologists when clinicians are uncomfortable interpreting imaging unsupported; we argue that incorporating such systems into routine clinical practice via MDTs offers additional advantages with regards to educational opportunities, recognition and time allocation for radiology support and possibly reduced stress resulting from fewer ad hoc discussion requests.
Malignancy bias
The two most diagnosed pathologies were lymphoproliferative and inflammatory disorders, representing 67.6% of patients. Both sensitivity and positive predictive value were lower in inflammatory disease at 31% and 56.3% respectively, compared with 72.5% and 74.4% in lymphoproliferative disease. Similarly, the diagnosis featured in the list of differentials of only 67.2% of patients with inflammatory disease, but 90% of those with lymphoproliferative disease. This may imply a ‘malignancy bias’, whereby clinicians recognise the importance of ruling out a malignant process as a top priority, given the greater likelihood for life-threatening disease compared with possible systemic inflammatory disorders, and document a differential diagnosis in keeping with this. This is supported by the recognised difficulty in distinguishing these two processes based on clinical or radiological features, even where attempts have been made to create protocols to do so [2,3,4].
Repeat biopsies
Repeat biopsies were undertaken in 10 patients during the study period. Indications for repeat biopsy were new symptoms/signs including mass (50%), relapsing disease or steroid dependence (30%), debulking excision biopsy (10%) and inconclusive histology with worsening signs (10%). 50% of repeat biopsies altered the diagnosis or management in some way, with 30% obtaining a specific diagnosis from previous non-specific inflammation (2 lymphomas, 1 granulomatosis with polyangiitis), 10% being given a more specific diagnosis (unspecified B cell lymphoma initially, MALT lymphoma latterly) and 10% ruling out recurrent malignancy (previous treated lymphoma, repeat biopsy only showed fibrosis). This highlights the importance of undertaking repeat biopsies where there is clinical concern, even in the context of previous conclusive histopathology results.
Documentation influences
Koukkoulli et al. noted that clinicians and radiologists did not offer a pre-operative diagnosis in 34.8% and 39.3% of patients, respectively [2], commenting that clinicians may be increasingly reliant upon investigations rather than their clinical acumen to make diagnoses. In our series, the rate where no diagnosis was offered was much lower at 18.3% of radiology reports and 10.3% for CR opinion; this reflects local practice of documenting a differential diagnosis during the orbital MDT. They also treated the documentation of more than one prebiopsy diagnosis as an incorrect diagnosis. However, there may be an array of influencing factors that affect documentation of a diagnosis or list of differential diagnoses. Whilst confidence in clinical or radiological diagnosis may be one such factor, others might include local protocols or preferences, experiences of preferences elsewhere, seniority of clinician, likely impact on management plan, and relationship with medicolegal aspects of documentation. Offering a list of differential diagnoses in order of suspected likelihood may be perceived as being more thorough, or a good educational adjunct for other department members, especially for trainees, or facilitate management when there are pooled surgical lists (in our centre, many orbital cases are pooled between two consultants to improve timely allocation of surgeries). Due to our habitual consideration of a list of differentials, we did not exclude patients for whom more than one differential diagnosis was offered but used the ‘top’ diagnosis for our analysis. This may have increased the overall ‘match rates’ compared with Koukkoulli et al.’s data but is reflective of real-world clinical practice.
Limitations
This was a retrospective case series, which is dependent on quality of documentation. Overall numbers are good, but sub-group analysis is not possible due to the small numbers of rarer pathologies such as peripheral nerve sheath tumours. Analysis has been undertaken under the belief that the order of documented differential diagnoses reflects the CR perception of likelihood, which may not be accurate in all circumstances (e.g. due to the ‘malignancy bias’ discussed above). Patients with orbital lesions who did not undergo orbital biopsy are not represented here – reasons for not biopsying include stable, minimally symptomatic lesions where CR diagnosis does not suggest sight or life-threatening disease (e.g. small cavernous haemangiomas), where pre-operative investigation reveals diffuse disease with another mass more accessible for biopsy, where patients fit a classical pattern of disease that is treated empirically (e.g. orbital myositis or thyroid eye disease), or where patients declined or were unable to have surgery. Patients are referred to our tertiary centre from hospitals across a wide region, so diagnoses in our cohort may not be truly representative of population incidence or prevalence.
Conclusion
Pre-operative diagnosis in patients undergoing orbital biopsy remains challenging, with substantial overlap in clinical and radiological features for the two most commonly biopsied orbital diagnoses of inflammatory disease and lymphoproliferative disorders. However, correct pre-surgical identification of orbital disorders is an important goal as it facilitates prioritisation of resources including theatre time, enables appropriate patient counselling regarding likely diagnosis, and guides management. There is limited data available comparing clinical and radiological pre-operative diagnoses with histopathological diagnosis, but this study and previous literature suggest a synergistic effect of combining radiological and clinical opinions, with increased accuracy when orbital surgeons and radiologists work together. Clinicians should be encouraged to provide all relevant clinical data alongside specific clinical questions when requesting radiological imaging to support high-quality radiological reports. Histopathological diagnosis remains the gold standard but repeat biopsy should be considered in patients where tissue diagnosis is non-diagnostic or does not correlate well with the clinical picture. Future developments in imaging technology may gradually reduce reliance on histopathological analysis, but our results demonstrate that clinic-radiological pre-biopsy diagnosis is still inaccurate in a significant proportion of cases even where expert clinicians work together.
MDTs incorporating specialist radiologists may offer reciprocal educational opportunities alongside the practical and diagnostic benefits of allocated time together for both specialties. The authors recommend that all orbital specialists have access to such MDTs, even if this is not feasible within their own unit; video conferencing technology can now facilitate discussions in a ‘hub and spoke’ model when appropriate.
Summary
What was known before
-
Orbital biopsy is the gold standard for diagnosis of orbital lesions.
-
Pre-operative diagnosis based on clinical or radiological findings is challenging, particularly differentiating between inflammatory and lymphoproliferative disorders.
-
Accurate pre-operative diagnosis supports effective use of resources, and can manage patient expectations.
What this study adds
-
Accuracy of clinical and radiological pre-operative diagnosis of orbital lesions are intrinsically linked due to their mutual reliance of high-quality information from the other.
-
Access to a regular multi-disciplinary team meeting including orbital surgeons and radiologists can offer reciprocal learning opportunities and improve pre-operative diagnostic accuracy.
References
Yilmaz K. The cognitive perspective on learning: its theoretical underpinnings and implications for classroom practices. Clearing House. 2011;84:204–12.
Koukkoulli A, Pilling JD, Patatas K, El-Hindy N, Chang B, Kalantzis G. How accurate is the clinical and radiological evaluation of orbital lesions in comparison to surgical orbital biopsy? Eye. 2018;32:1329–33.
Bacorn C, Gokoffski KK, Lin LK. Clinical correlation recommended: accuracy of clinician versus radiologic interpretation of the imaging of orbital lesions. Orbit. 2020;13:1–5.
Hunt S, Pereni I, Ford R, Garrott H. Lymphoma and inflammatory disorders presenting in the orbit– a comparison of characteristics from a 10-year series in a tertiary hospital. Orbit. 2021;1–6 https://doi.org/10.1080/01676830.2021.1892771.
Author information
Authors and Affiliations
Contributions
SVH – data collection and analysis and article writing. IP – data collection and support with data analysis and article revision. MW – article concept, interpretation of radiological images and article revision. RF – article concept, support with data analysis and article revision. HG – article concept, support with data analysis and article revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hunt, S.V., Pereni, I., Williams, M. et al. Multi-disciplinary team meetings with specialist radiologists may improve pre-operative clinico-radiological diagnostic accuracy in patients requiring orbital biopsy and offer reciprocal educational opportunities. Eye 36, 2200–2204 (2022). https://doi.org/10.1038/s41433-021-01834-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01834-1