Abstract
Background
In the wake of the controversy surrounding the SYMPLICITY HTN-3 trial and data from subsequent trials, this review aims to perform an updated and more comprehensive review of the impact of renal sympathetic denervation on cardiac arrhythmias.
Methods and results
A systematic search was performed using the Medline, Scopus and Embase databases using the terms “Renal Denervation” AND “Arrhythmias or Atrial or Ventricular”, limited to Human and English language studies within the last 10 years. This search yielded 19 relevant studies (n = 6 randomised controlled trials, n = 13 non-randomised cohort studies) which comprised 783 patients. The studies show RSD is a safe procedure, not associated with increases in complications or mortality post-procedure. Importantly, there is no evidence RSD is associated with a deterioration in renal function, even in patients with chronic kidney disease. RSD with or without adjunctive pulmonary vein isolation (PVI) is associated with improvements in freedom from atrial fibrillation (AF), premature atrial complexes (PACs), ventricular arrhythmias and other echocardiographic parameters. Significant reductions in ambulatory and office blood pressure were also observed in the majority of studies.
Conclusion
This review provides evidence based on original research that ‘second generation’ RSD is safe and is associated with reductions in short-term blood pressure and AF burden. However, the authors cannot draw firm conclusions with regards to less prominent arrhythmia subtypes due to the paucity of evidence available. Large multi-centre RCTs investigating the role of RSD are necessary to comprehensively assess the efficacy of the procedure treating various arrhythmias.
Graphic abstract

Similar content being viewed by others
Introduction
Cardiac arrhythmias contribute significantly to morbidity and mortality worldwide [1, 2]. Atrial arrhythmias are the commonest arrhythmias observed in clinical practice with an increasing prevalence due partly to an ageing population, with atrial fibrillation (AF) alone affecting 33.5 million people globally [1]. AF is additionally linked with strokes which can be highly debilitating [3, 4]. By contrast, ventricular arrhythmias are less common but are associated with sudden cardiac death [5], affecting around 184,000–450,000 individuals per year in the United States of America [2, 6, 7].
Arrhythmias are complex biophysical phenomena, and despite significant advances in medical technology over the past decades, current medical and invasive management options cannot abolish their occurrences completely. Renal sympathetic denervation (RSD) was first performed in 1925 [8], but due to its inability to reduce blood pressure safely as well as effectively in patients with resistant hypertension, did not gain significant attention until the recent consequential findings of the HTN-1 [9] and HTN-2 [10] trials. Given that hypertension is one of the most important risk factors for developing AF and its recurrence [11], there has been speculation that RSD may reduce AF burden [12, 13]. Whilst this may be a direct consequence of BP reduction, there is some evidence that the impact of RSD on cardiac arrhythmias may be independent of BP effects [14]. RSD has also proven to have a role in several related conditions in which the autonomic nervous system plays an important role, such as heart failure, chronic kidney disease (CKD) and glucose metabolism [8, 15]. Thus, RSD empirically has a plausible biological mechanism to affect the autonomic nervous system, feedback onto the cardiovascular system, and together with early studies [14] hinting at a possible antiarrhythmic effect. Thus, the results of HTN-3 trial [16] that found that both the RSD and control groups showed similar BP reductions at 6 months, do not directly affect the potential of RSD in treating cardiac arrhythmias. The data from the HTN-3 [16] trial have been reported to be controversial for the following reasons: (1) medication adherence (2) suboptimal procedure performance, (3) patient selection and (4) operator experience [17,18,19]. Since then, there have been many studies such as the DENERHTN trial [20], the SPYRAL HTN-OFF MED trial [21], RADIANCE-HTN SOLO trial [22] and SPYRAL HTN-ON MED [23] trial that report a significant reduction in BP.
In 2016, a systematic review examined the effects of RSD on cardiac arrhythmias [14]. However, since then, additional trials have been completed and published. Moreover, the review did not examine all domains relevant to RSD and cardiac arrhythmias. For example, whilst there is a relatively larger body of evidence on the short- and long-term effects of RSD on patients with AF and hypertension, the effects of RSD on AF in normotensive patients or on ventricular arrhythmias have yet to be fully investigated. In this study, we aim to perform an updated and comprehensive systematic review on the effects of RSD on both atrial and ventricular arrhythmias.
Methods
Search strategy
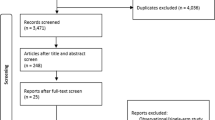
This review used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Medline, Scopus and Embase were searched from January 2010 to October 2020 using search terms “Renal Denervation” AND “Arrhythmias or Atrial or Ventricular”. Searches were limited to that of original research and human studies in the English language. One reviewer (KN) independently screened the titles and abstracts of the results obtained from the search strategy. From the initial 1192 papers initially identified by the search strategy (Fig. 1), 44 relevant studies were selected and full texts obtained. Three independent reviewers (NNK, KN and KJ) then independently studied full texts to include and exclude relevant studies based on the exclusion criteria defined in the protocol. The reference lists of these studies were studied to identify other relevant studies to be included in the studies. Duplicates were also screened for and removed as appropriate. Any discrepancies were discussed and resolved internally with the senior author (KJ).
Extraction
Extraction was then conducted by one of the reviewers (NNK) onto a standardised excel extraction sheet independently. The reviewer extracted all outcomes listed in the inclusion criteria. Two other reviewers (KN and KJ) independently checked the extracted data with the original full texts.
Inclusion criteria
We included studies that were (1) original research study, i.e. randomised controlled trial (RCT) or non-randomised observational studies (retrospective or prospective cohort), (2) studied a human population with any form of cardiac arrhythmia, (3) had a treatment arm where RSD was used, (4) reported outcomes as follows: arrhythmia (atrial or ventricular) recurrence, changes in blood pressure, change in heart rate, mortality, hospitalisation, complications (general or procedural), renal function and cardiac morphological changes (echocardiographic parameters).
Results
Search results and study characteristics
The search was conducted on the fourth of October 2020 and yielded a total of 1199 results across the Ovid Medline, Scopus and Embase databases. 1078 studies were excluded after screening of titles and abstracts due to irrelevant study design or topic. Of the remaining n = 121 studies, n = 77 were excluded (n = 29 due to irrelevant outcomes, n = 14 irrelevant population, n = 11 irrelevant study design or had no results published at the time of search, n = 9 case reports, n = 6 duplicates, n = 1 study was terminated early, and n = 1 erratum). Forty-four full-text studies were obtained and assessed by 2 reviewers (NNK, KN). A further 25 studies were excluded as duplicates, resulting in a total of 19 studies included in this review (Fig. 1). These 19 studies comprised 6 randomised controlled trials (RCTs) and 13 were non-randomised cohort studies. The population across all 19 studies was 783 participants of which 505 (64.5%) were male and 278 (35.5%) were female. The age across the studies included ranged from 47 to 81 years (Table 1). Risk of bias was assessed using the Risk of Bias in Non-randomised Studies—of Interventions (ROBINS-I) tool for non-randomised studies (Fig. 2) and the Revised Cochrane risk of bias tool for randomised trails (RoB-2) for randomised trials (Fig. 3).
Recurrence of atrial arrhythmias
Fourteen studies included atrial arrhythmia outcomes such as AF, atrial flutter, premature atrial contractions (PACs), supraventricular ectopic beats (SVEs), atrial premature complexes and premature supraventricular contractions. Thirteen studies studied concurrent RSD (with or without PVI), and 1 [24] of the studies being crossover/RSD only. At 12 months post-RSD, eight studies report recurrence of AF as “freedom from AF”, “AF-free”, “total AF episodes” and “AF burden (min/day).” Of the 18 studies that investigated the recurrence of arrhythmias (atria and ventricle) post-RSD, the majority of used 24-Holter monitors (n = 9) to capture the episode of recurrence at defined time points. Four studies (n = 4) used implantable cardiac defibrillators (ICDs), one study (n = 1) used an implantable cardiac monitor, one study (n = 1) used ECG, one study (n = 1) used a pacemaker, one study (n = 1) used “electrophysiology studies” and one study (n = 1) used a 7-day Holter monitor.
However, it is worth nothing that one of these studies reports AF recurrence which is not exclusively 12 months and 22.4 ± 12.1 months was the follow-up period [25]. In these eight studies, six of them compared pulmonary vein isolation in the treatment of AF with pulmonary vein isolation and RSD. In the remaining two studies, the only invasive intervention was the RSD itself (Table 1). All studies used Holter monitoring to detect the presence of AF at the pre-defined follow-up points (from 1 to 12 months) though the total time under surveillance was different. Four of these studies used Holter monitoring varying from 24 h to 7 days at the pre-defined intervals to detect AF. The remaining studies used implantable cardiac monitors to continuously monitor for arrhythmias or used implantable pacemakers to detect arrhythmias. Four studies reported “freedom from AF” at 12 months as 61% (n = 20), 76% (n = 16), 69% (n = 9) and 63% (n = 26), respectively [26,27,28,29]. All studies, whether RSD alone or RSD with PVI, report a significant (p < 0.05) or marked numerical decrease in AF recurrence, or a higher number of patients that are AF free in the treatment arm that includes RSD as opposed to the control group [25,26,27,28,29,30,31,32]. In addition to freedom from AF, Steinberg et al. [1] reported freedom from atrial flutter and tachycardia at 12 months that was significantly higher in the RSD treatment group (p = 0.006).
The remaining studies looked into outcomes of various other types of rhythm abnormalities with significant findings. Ukena et al. [33] and Schirmer et al. reported a significant decrease in PACs at 6 months. Feyz et al. [31] reported a numerical decrease in SVEs at 6 and 12 months post-RSD. Some studies looked at more short-term outcomes for various atrial arrhythmias. Qiu et al. [34] reported that RSD was postulated to have improved rate control in patients with persistent AF at 7 days post-procedure. McLellan et al. [35] reported there not to be any change at 7 days post-RSD with regards to PACs and atrial tachyarrhythmias. Tsioufis et al. [36] reported a significant decrease of premature supraventricular contractions at 1 and 6 months post-RSD.
Recurrence of ventricular arrhythmias
Nine studies investigated the impact of RSD on different ventricular arrhythmia outcomes, such as premature ventricular contractions (PVCs), ventricular ectopic beats (VE), ventricular rate response (VRR), “median ventricular arrhythmia episodes per month” that included episodes of electrical storm (ES) [37], polymorphic premature ventricular complexes (PVCs), burden or episodes of ventricular tachycardia (VT) and ventricular fibrillation (VF). Three studies report that various subtypes of ventricular arrhythmias remained unchanged post-RSD at different time points (7 days, 6 months and 12 months) [31, 33, 35]. However, Feyz et al. [31] reported a significant decrease in VRR during AF at 6 and 12 months, and Jiang et al. [37] reported a significant decrease in media ventricular arrhythmia episodes per month. Kiuchi et al. [24] reported a significant decrease in PVCs at 1 and 6 months after RSD. Evranos et al. [38] report a significant decline in burden of VT and VF at 3, 6, 12 and 18 months. Ukena et al. [39] in an earlier study also report a significant decrease in VT and VF at 1 and 3 months. Armaganijan et al. [40] report a reduced median number of VT and VF at 1 and 6 months. Tsoufis et al. [36] report a significant decrease in PVC and “ventricular arrhythmias” at 1- and 6-month follow-ups (Table 2).
Changes in heart rate
Eleven studies reported the effects of RSD on HR at various time points after RSD. Six trials reported HR to have remained unchanged or have a non-significant change at 7 days and 1, 6 and 12 months post-RSD [25, 31, 33, 37, 39, 40]. However, in a trial by Ukena et al. [33], there is a subgroup of patients with a baseline HR of > 72 bpm, that have been reported to have had a significant reduction in HR at 6 months. Similarly, there are five other studies that have documented a significant decrease in HR post-RSD at various time points during follow-up (7 days, 1, 3 and 6 months) [24, 34, 36, 41, 42]. Furthermore, Kiuchi et al. [24] reported a numerical decrease in HR at 1 and 6 months post-RSD (Table 3).
Blood pressure (BP) changes
All 19 trials investigated the change in BP of patients after undergoing RSD at various time points throughout follow-up. BP measurements were either “office BP,” “Ambulatory or 24-h BP” or “mean systolic and diastolic BP”. Two studies reported no significant change in BP 3 months post-RSD [24, 27] but Ukena et al. [42] report a significant decrease in BP at 3 months. Nine studies recorded BP in patients 6 months post-RSD, of which five studies [26, 31, 36, 41, 42] reported a significant decrease in blood pressure; however, four studies reported no significant change from baseline [25, 27, 37, 40]. Two studies report BP outcomes at 9 months, both of which were reported to have seen a significant decrease of BP at that time point [28, 29]. At 12 months post-RSD, five studies reported a significantly lowered blood pressure [26, 28, 30,31,32]; however, two studies reported no significant change in blood pressure at that time point [24, 27].
As well as the standard follow-up schedule outlined above followed by a large plurality of studies, Tsioufis et al. [36] investigate a more short-term outcome and have found that RSD reduces BP significantly 1 month post-RSD despite Ukena et al. [39] reporting no significant change in BP after 1 month. Qiu et al. [34] report no change in BP at 7 days post-RSD. Evranos et al. [38] investigate BP beyond 12 months and report no change in BP at 15 months (Table 3).
Renal outcomes
A total of seven included studies reported renal outcomes pre- and post-RSD [24,25,26,27,28, 31, 37, 38]. Seven studies recorded the estimated glomerular filtration rate (eGFR) [24,25,26,27,28, 31, 38]. Three studies [24, 25, 31] showed a significant increase (p < 0.05 or p < 0.001) in eGFR. All three of these studies reported significant increase in eGFR at 6 months post-RSD [24, 25, 31] and two studies reported a significant increase (p < 0.05 or p < 0.001) in eGFR at 12 months [25, 26]. Four studies reported a numerical increase or unchanged eGFR upon follow-up [27, 28, 31, 38]. The albumin to creatinine ratio (ACR) was reported in four studies [24, 25, 27, 31], of which three studies reported a significant decrease (p < 0.05 or p < 0.001) in the ACR [24, 25, 31] and a single study, Kiuchi et al. [27] reported a numerical decrease in the ACR at follow-up. Creatinine levels were reported in five studies [24,25,26,27, 37]. Three studies [24, 25, 31] reported a significant decrease (p < 0.05 or p < 0.001) in creatinine and a further two reported numerical decreases at 6 months [37] and 12 months [27] (Table 4).
Complications, hospitalisation and quality of life
Fourteen included trials investigated the complications (general and procedure related) post-procedure. Ten studies report no complication following RSD. Three studies specifically stated the absence of “procedure related complications” [26, 28, 29] and the remaining seven studies state the absence of “complications” [24, 25, 27, 34, 37,38,39]. However, Steinberg et al. [30] reported seven procedural complications and Feyz et al. [31] reported one procedural complication. Although Armaganijan et al. [40] reports “no major complications”, one patient developed severe bradycardia during the procedure. Ukena et al. [42] reported 10 complications in their RSD treatment group, that included eight transient vagal reaction during RSD and two pseudoaneurysms of the femoral artery.
Only five studies collected data regarding hospitalisation post-RSD (Table 5). Four studies report that all their patients were hospitalised 24 h post-RSD [24,25,26,27]. Steinberg et al. [30] reported that a total of eight patients (5.2%) were hospitalised post-procedure for atrial fibrillation-related symptoms (n = 5), cardiac or vascular surgery (n = 2) and due to myocardial infarction (n = 1). Only Feyz et al. [5] report quality of life (QoL) to be significantly improved at 6 and 12 months following RSD.
Mortality
Of the seven studies that report death post-RSD, three studies report no death at all [25, 31, 37]. However, of the three, Feyz et al. [31] only report “no cases of cardiovascular death”. Steinberg et al. [30] and Evranos et al. [38] report two (none related to ablation) and one (decompensated heart failure) death(s) in their RSD groups, respectively. Ukena et al. [39] reports three deaths within 1 year of RSD due to progressive heart failure (n = 2) at 6 and 7 months post-RSD, and septic shock with respiratory failure (n = 1) 2 months post-RSD. Lastly, Armaganijan et al. [40] report three cases of death but none was attributable to ventricular arrhythmias (Table 5).
Discussion
The role of RSD in attenuating cardiac arrhythmias lies in the link between the renal afferent and efferent sympathetic nervous system (SNS) fibres that modulate the central nervous system control of cardiac electrophysiological properties. The SNS increases HR and increases atrioventricular conduction which is potentially arrhythmogenic. RSD operates by dampening pathological SNS overstimulation. Moreover, the SNS innervates the kidneys, playing a major role in the pathophysiology of resistant hypertension via the renin–angiotensin–aldosterone system (RAAS) and sodium absorption. This supports the findings of recent studies that suggest this autonomic overdrive plays an important aetiological role in many cardiac arrhythmias such as AF and potentially an explanation of why it often coexists with hypertension. Recent evidence suggests that an overdrive of the SNS contributes to the “perception” of paroxysmal symptomatic AF episodes and hence it is promulgated that the dampening of SNS activity not only decreases BP but also may decrease the occurrence of such “perceived” arrhythmias particularly paroxysmal AF [43, 44]. Given that the autonomic nervous system innervates both atria and ventricles, it is postulated that RSD might have a role in treating arrhythmias of both atrial (i.e. AF) and ventricular origin (i.e. VT or VF) [45].
Hypertension has been shown to be the most relevant risk factor in the development of AF, which is itself the most common type of arrhythmia in the United Kingdom and set to grow in prevalence as the population ages [46, 47]. The link between the SNS activity, cardiac arrhythmias and hypertension is well documented and has been a target of interest for much research for numerous pharmacological and non-pharmacological interventions [48, 49]. Furthermore, first-line pharmacological treatments for hypertension such as angiotensin-converting enzyme (ACEi) inhibitors and angiotensin receptor blockers (ARBs) are also noted for their use in patients suffering from AF [48]. However, poor drug adherence [50] as well has the increased prevalence of treatment-resistant hypertension, a single intervention with long-term efficacy is seen as an increasingly attractive option for healthcare providers, researchers and patients. RSD has a controversial history regarding its use to lower BP of patients due to the findings of the SYMPLICITY HTN-3 trial [16]. However, redesigned so called ‘second generation’ studies such as SPYRAL HTN -ON, SPYRAL HTN-ON and RADIANCE SOLO have reported that RSD has been shown to reduce Office and Ambulatory BP in patients for up to 12 months [51]. However, though RSD has been shown to be effective in the short term (up to 12 months), there is still a paucity of data on the long-term outcomes of hypertensive patients. The largest study on the longer term outcome is the is the Global Simplicity Registry (GSR) a multi-centre study, which has reported data on more than 2500 patients up to 3 years post-procedure [52].
These extensive studies have, however, shown that RSD is a safe procedure for patients with no significant complications with sham or control groups. The majority of the studies in this systematic review had no complications. There was no mortality directly attributable to the procedure itself in any of the studies. Of note is the large variation in reporting of complications between the studies—the majority of complications were reported in two studies Steinberg et al. [30] and Ukena et al. [42]. The majority of complications were, however, benign with vasovagal effects being predominant. Only one arrhythmic complication (severe bradycardia) was seen, underlining the link between the SNS and cardiac electrophysiology.
Another safety concern surrounding RSD is the potentially deleterious effects on renal function. However, the safety of renal denervation has been shown in multiple studies such as Hering et al. [53] and Prasad et al. [54] where both found no significant alterations in renal function in patients with stage 3–4 kidney disease after renal denervation up to 12 and 24 months, respectively. Furthermore, with RSD, there was no significant difference in mortality or adverse outcomes in the studies that could be attributed to ventricular arrhythmias or the renal denervation procedure itself.
Quality of life scores, though initially may seem disappointing, likely do not reflect an accurate representation of the efficacy RSD on quality of life for cardiac rhythm disturbances—many of the trials were primarily driven for HTN and not for cardiac rhythm disturbances. The pre-existent rhythm disturbances may not be significantly impacting upon the baseline QoL making improvements hard to detect. HTN itself is largely asymptomatic, and therefore, QoL improvements are likely to be driven by reduced medication needs and contact with medical services in the studies where this was the primary end point.
The RSD procedure itself was technically more comparable. Similar radiofrequency energy use across the studies helps in comparison. Anticoagulation peri-procedurally was also largely with unfractionated heparin. However, ablation time was more variable (only one study used ablation < 30 s, all others > 60 s, though a significant number > 120 s). Thus, from a technical aspect, the largest point of difference is the ablation time (and therefore, total energy delivered) as well as the equipment used to deliver the energy. Ablation time may simply reflect operator familiarity with the anatomy to faithfully deliver the ablation to the target.
The effect of RSD on arrhythmic outcomes seems to be more pronounced. The atrial arrhythmia of primary concern in the studies was AF, with a secondary focus on atrial flutter. Flutter outcomes were not commented on between the two arms apart form in one study where there was a significant decrease in recurrence of atrial flutter. This is not unexpected as of the eight studies that measured outcomes RSD with concurrent ablation for AF/flutter, and all the protocols included empirical cavo-tricuspid isthmus line ablation if the patient had a history of atrial flutter, a procedure with a high success rate without adjunctive therapy. Therefore, it would not be expected to see significant recurrence, and therefore, reduction in recurrence of flutter regardless if RSD had been performed or not.
The outcomes for AF are worth considering carefully. Six studies measured pulmonary vein isolation (PVI) and compared it to PVI and RSD for the treatment of AF. The majority of these studies were in patients with a history of paroxysmal AF, a patient population in which PVI has greater success. However, two studies included patients with episodes of persistent AF as well as paroxysmal AF. Permanent AF was excluded, as by definition, these patients are not to be restored to sinus rhythm. In all the studies, there was a significant improvement in freedom from AF with adjunctive RSD treatment with PVI. Furthermore, the detection of AF was usually done with Holter monitoring, though at least half the studies used implantable devices to continuously monitor cardiac rhythms, which will improve detection rates of arrhythmias dramatically. The remaining four studies used Holter monitoring varying in duration from 24 h at prespecified times up to the 12-month follow-up period to 7 day Holter monitoring. In all these studies, there was statistically significant improvement in freedom from AF. The improvement in outcomes was consistent despite large variation in the ability to detect arrhythmias (i.e. the total time where surveillance for AF was conducted was variable between studies) suggesting a real effect rather than one driven by poor detection.
A plurality of studies documented similar improvements in PACs (premature atrial complexes) though this was not universal. Improvements in the ventricular response rates of patients with persistent AF were also noted. The results for ventricular arrhythmias in patients who underwent RSD were similarly poignant, with six of nine studies showing a reduction in ventricular arrhythmias of all kinds following RSD. In the three studies that showed no such effect, the total surveillance time was very low—Ukena [33] had a single 24-h Holter detection period at one follow-up point (6 months post-procedure) whilst McLellan [35] had a 7-day surveillance period at 6 months post-procedure. It is entirely feasible that this is not a long enough surveillance time to detect statistically significant changes in ventricular arrhythmias, especially considering the relative lower frequency of ventricular compared to atrial arrhythmias. The 6-month follow-up point to measure outcomes may initially seem shorter than the 12 months in other studies, though should be long enough to detect changes induced by ventricular remodelling according to the body of the literature in other pathologies where ventricular remodelling is observed such as heart failure. Thus, there seems to be a somewhat conflicting evidential basis for reductions in ventricular arrhythmic burden with RSD. Future studies will have to more carefully define the patient population enrolled into the studies and perhaps concentrate on patient with known IHD and higher burden of ventricular arrhythmias from the outset—this will allow detection of a change in ventricular arrhythmic burden in the patient population that is most at risk and allow studies to detect smaller changes with greater power.
The effect on heart rate is more mixed, with no clear consensus emerging. This may be due to large confounds such as with antiarrhythmic and anti-hypertension medication that may alter heart rate, a whole range of other physiological correlates including co-morbidities as well as the quality of the studies themselves.
There seems to be indications for the efficacy for RSD for blood pressure control with the majority of studies showing statistically lower blood pressure from 6 months onwards in both an ambulatory and office setting. BP control at earlier time points (1–3 months post-RSD) and long-term outcomes of BP control with RSD have not been extensively investigated with only one study investigating outcomes beyond 12 months. The effect of this improved control on pharmacotherapy cannot be determined due to the large variability in reporting in the studies, with in depth dosage and treatment regimens before and after RSD not being available to perform a comparison.
End-organ effects of RSD were explored in terms of both renal and cardiac outcomes. Renal denervation therapy has been used as an invasive but safe procedure to treat heart failure [55]. Importantly, a number of studies reported that RDN improves left ventricular ejection fraction (LVEF), an important prognostic marker and determinant of clinical management. Further echocardiographic measures such as septal wall thickness and ventricular mass indices were also reduced in a number of studies. However, there is conflicting evidence with some studies reporting no such effect. More trials need to be conducted to follow-up on these findings. Similarly, the lack of large and reliable studies was noted as an issue in Fukuta et al. [56], which noted that RSD was associated with a statistically significant increase in LV function (including LVEF) as well as a broader improvement [56]. Similarly, there is conflicting evidence for improvement in eGFR, though reassuringly there is no evidence for RSD being associated with deterioration in renal function even in patients with chronic kidney disease.
Pulmonary vein isolation (PVI) is used widely to treat symptomatic AF; however, its success rate is not optimal and there is a marked recurrence rate of arrhythmia is certain subgroup of patients. However, with the addition of RSD to PVI, the results of several RCTs and meta-analysis seem promising with regards to lowering the rate of AF recurrence significantly [11, 30, 57]. Studies have reported a role for RSD in other atrial and ventricular arrhythmia subtypes, and the majority of studies here demonstrate reduced ventricular arrhythmias. Importantly, however, these studies were not adequately controlled for concomitant antiarrhythmic drug use and a large number of patients were taking beta-blockers in the studies. Further studies are warranted to be able to draw firm conclusions.
In our analysis, it has proven difficult to pool results across studies with regards to the outcomes of RSD largely due to two reasons: (1) the small population size in studies. As RSD has yet to be performed as a standard treatment for arrhythmias, large, randomised studies have not been easy to conduct and the logistically feasible alternatives such as cohort studies in real-world settings have proven useful for investigating different outcomes for RSD. Arrhythmia outcomes in RSD studies often rely on subgroup analysis which are often underpowered with small sample sizes. (2) Large heterogeneity in procedural protocol, treatment regimen, care pathway and RSD equipment. Namely, the majority of studies used RSD in combination with PVI on the treatment arm, and therefore, highlighting the scarcity of evidence of the implications of using RSD in isolation for arrhythmias. Therefore, treatment outcomes across several institutions cannot be compared without acknowledging the fact that the differences mentioned have a role to play in patient outcomes.
This systematic review has several limitations that stem from the quality of studies included. (1) The review included a large number of unblinded, non-randomised observational studies. Data extracted from these cohort studies are potentially affected by biases due to the study design such as selection bias and allocation bias. (2) The generalisability and statistical power of the data is questionable given that the studies mostly do not have a large study population, some as few as 8. (3) The studies were also not powered sufficiently for us to perform subgroup analysis on the outcomes. (4) Most studies include patients with hypertension and form of arrhythmia, i.e. AF. Therefore, it can be argued that the hypothesis drawn based on hypertension patients cannot be extrapolated to patients without hypertension. (5) In some patients, antihypertensives and antiarrhythmic were used alongside RSD, and therefore, could have potentially influenced the true outcome of the procedure. (6) In some studies, the RSD procedure most notably total ablation power delivered, was not standardised and could have an impact on the efficacy of the treatment and outcomes. (7) The patient population in some trials are heterogenous and propensity scores were not matched to minimise confounding factors. (8) The inclusion of several types of arrhythmia as an outcome did not allow us to interpret the outcomes of each arrhythmia individually for a minuscule portion of the analysis. Lastly, the reporting of arrhythmia recurrence was variable which made it difficult for us to pool data and to quantify the difference from various studies for analysis.
To gain a more concrete and directional understanding on the true effects of RSD on cardiac arrhythmias, future studies should be conducted to investigate further the potential clinical efficacy, safety and benefits in arrhythmia patients in large-scale RCTs. These studies should be arrhythmia specific and have long-term outcome measures that go beyond a year post-RSD. The focus of these studies should initially be on AF patients with resistant hypertension as this is currently a major issue in clinical practice. Such studies may produce sufficient data, that might enable the safe, efficacious and minimally invasive RSD to be incorporated into clinical guidelines permitting its use in everyday clinical practice. Consensus on the most effective RSD protocols is also needed, therefore, enabling further research to be done on a multi-centre and international scale. With uniformity in the deliverance of RSD, more direct comparisons could be made, leading to a further therapeutic option in the clinician’s arsenal.
The role of RSD in attenuating AF is becoming more widely accepted when used with PVI [26, 28,29,30,31,32, 34]. Its potential in treating other arrhythmias has yet to be established but the evidence published till date report promising results.
Availability of data and material (data transparency)
NA.
Code availability (software application or custom code)
NA.
References
Mujer MT, Al-Abcha A, Saleh Y, Nerusu LA, Boumegouas M, Herzallah K et al (2020) Effect of combined renal denervation and pulmonary vein isolation in atrial fibrillation recurrence in hypertensive patients: a meta-analysis. Pacing Clin Electrophysiol. https://doi.org/10.1111/pace.14009(Epub 2020/07/08, PubMed PMID: 32638388)
Khurshid S, Choi SH, Weng LC, Wang EY, Trinquart L, Benjamin EJ et al (2018) Frequency of cardiac rhythm abnormalities in a half million adults. Circ Arrhythm Electrophysiol 11(7):e006273. https://doi.org/10.1161/CIRCEP.118.006273(PubMed PMID: 29954742; PubMed Central PMCID: PMC6051725)
Korantzopoulos P, Letsas K, Fragakis N, Tse G, Liu T (2018) Oxidative stress and atrial fibrillation: an update. Free Radic Res 52(11–12):1199–1209. https://doi.org/10.1080/10715762.2018.1500696(Epub 2018/07/14, PubMed PMID: 30003814)
Lindberg T, Wimo A, Elmståhl S, Qiu C, Bohman DM, Sanmartin BJ (2019) Prevalence and incidence of atrial fibrillation and other arrhythmias in the general older population: findings from the Swedish National Study on aging and care. Gerontol Geriatr Med. 5:2333721419859687. https://doi.org/10.1177/2333721419859687(Epub 2019/06/27, PubMed PMID: 31276022; PubMed Central PMCID: PMC6598326)
Tse G, Yan BP (2017) Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace 19(5):712–721. https://doi.org/10.1093/europace/euw280(Epub 2016/10/06, PubMed PMID: 27702850)
Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M et al (2008) Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 51(3):213–228. https://doi.org/10.1016/j.pcad.2008.06.003(PubMed PMID: 19026856; PubMed Central PMCID: PMC2621010)
Roberts-Thomson KC, Lau DH, Sanders P (2011) The diagnosis and management of ventricular arrhythmias. Nat Rev Cardiol 8(6):311–321. https://doi.org/10.1038/nrcardio.2011.15(Epub 2011/02/22, PubMed PMID: 21343901)
Olsen LK, Kamper AL, Svendsen JH, Feldt-Rasmussen B (2015) Renal denervation. Eur J Intern Med 26(2):95–105. https://doi.org/10.1016/j.ejim.2015.01.009(Epub 2015/02/14, PubMed PMID: 25676808)
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K et al (2009) Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373(9671):1275–1281. https://doi.org/10.1016/S0140-6736(09)60566-3(Epub 2009/03/28, PubMed PMID: 19332353)
Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M et al (2010) Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376(9756):1903–1909. https://doi.org/10.1016/S0140-6736(10)62039-9(Epub 2010/11/17, PubMed PMID: 21093036)
Pranata R, Vania R, Raharjo SB (2020) Efficacy and safety of renal denervation in addition to pulmonary vein isolation for atrial fibrillation and hypertension-Systematic review and meta-analysis of randomized controlled trials. J Arrhythm. 36(3):386–394. https://doi.org/10.1002/joa3.12353(Epub 2020/04/27, PubMed PMID: 32528562; PubMed Central PMCID: PMC7279983)
Verdecchia P, Angeli F, Reboldi G (2018) Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res 122(2):352–368. https://doi.org/10.1161/CIRCRESAHA.117.311402 (PubMed PMID: 29348255)
Dzeshka MS, Shantsila A, Shantsila E, Lip GYH (2017) Atrial fibrillation and hypertension. Hypertension 70(5):854–861. https://doi.org/10.1161/HYPERTENSIONAHA.117.08934(Epub 2017/09/11, PubMed PMID: 28893897)
Bazoukis G, Korantzopoulos P, Tsioufis C (2016) The impact of renal sympathetic denervation on cardiac electrophysiology and arrhythmias: a systematic review of the literature. Int J Cardiol 220:87–101. https://doi.org/10.1016/j.ijcard.2016.06.043(Epub 2016/06/14, PubMed PMID: 27372050)
Akinseye OA, Ralston WF, Johnson KC, Ketron LL, Womack CR, Ibebuogu UN (2020) Renal sympathetic denervation: a comprehensive review. Curr Probl Cardiol. https://doi.org/10.1016/j.cpcardiol.2020.100598(Epub 2020/05/03, PubMed PMID: 32448758)
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT et al (2014) A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370(15):1393–1401. https://doi.org/10.1056/NEJMoa1402670(Epub 2014/03/29, PubMed PMID: 24678939)
Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M et al (2015) Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 36(4):219–227. https://doi.org/10.1093/eurheartj/ehu441(Epub 2014/11/16, PubMed PMID: 25400162; PubMed Central PMCID: PMC4301597)
Mahfoud F, Schmieder RE, Azizi M, Pathak A, Sievert H, Tsioufis C et al (2017) Proceedings from the 2nd European clinical consensus conference for device-based therapies for hypertension: state of the art and considerations for the future. Eur Heart J 38(44):3272–3281. https://doi.org/10.1093/eurheartj/ehx215(PubMed PMID: 28475773; PubMed Central PMCID: PMC5837218)
Kiuchi MG, Esler MD, Fink GD, Osborn JW, Banek CT, Böhm M et al (2019) Renal denervation update from the international sympathetic nervous system summit: JACC state-of-the-art review. J Am Coll Cardiol 73(23):3006–3017. https://doi.org/10.1016/j.jacc.2019.04.015 (PubMed PMID: 31196459)
Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P et al (2015) Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 385(9981):1957–1965. https://doi.org/10.1016/S0140-6736(14)61942-5(Epub 2015/01/26, PubMed PMID: 25631070)
Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA et al (2017) Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390(10108):2160–2170. https://doi.org/10.1016/S0140-6736(17)32281-X(Epub 2017/08/28, PubMed PMID: 28859944)
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J et al (2018) Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 391(10137):2335–2345. https://doi.org/10.1016/S0140-6736(18)31082-1(Epub 2018/05/23, PubMed PMID: 29803590)
Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S et al (2018) Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 391(10137):2346–2355. https://doi.org/10.1016/S0140-6736(18)30951-6(Epub 2018/05/23, PubMed PMID: 29803589)
Kiuchi MG, Silva GR, Paz LM, Chen S, Souto GL (2016) Proof of concept study: renal sympathetic denervation for treatment of polymorphic premature ventricular complexes. J Interv Card Electrophysiol 47(2):221–229. https://doi.org/10.1007/s10840-016-0146-1(Epub 2016/05/30, PubMed PMID: 27240438)
Kiuchi MG, Chen S, E Silva GR, Rodrigues Paz LM, Kiuchi T, de Paula Filho AG et al (2017) The addition of renal sympathetic denervation to pulmonary vein isolation reduces recurrence of paroxysmal atrial fibrillation in chronic kidney disease patients. J Interv Card Electrophysiol 48(2):215–222. https://doi.org/10.1007/s10840-016-0186-6(Epub 2016/10/04, PubMed PMID: 27704317)
Kiuchi MG, Chen S, Hoye NA, Purerfellner H (2018) Pulmonary vein isolation combined with spironolactone or renal sympathetic denervation in patients with chronic kidney disease, uncontrolled hypertension, paroxysmal atrial fibrillation, and a pacemaker. J Interv Card Electrophysiol 51(1):51–59. https://doi.org/10.1007/s10840-017-0302-2(Epub 2017/12/22, PubMed PMID: 29264729)
Kiuchi MG, Chen S, Silva GR, Paz LM, Kiuchi T, de Paula Filho AG et al (2016) Pulmonary vein isolation alone and combined with renal sympathetic denervation in chronic kidney disease patients with refractory atrial fibrillation. Kidney Res Clin Pract 35(4):237–244. https://doi.org/10.1016/j.krcp.2016.08.005(Epub 2016/09/08, PubMed PMID: 27957419; PubMed Central PMCID: PMC5142261)
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A et al (2012) A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 60(13):1163–1170. https://doi.org/10.1016/j.jacc.2012.05.036(Epub 2012/09/11, PubMed PMID: 22958958)
Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Bayramova S, Losik D et al (2014) Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: early experience. Heart Rhythm 11(7):1131–1138. https://doi.org/10.1016/j.hrthm.2014.03.055(Epub 2014/04/03, PubMed PMID: 24691452)
Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E et al (2020) Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA 323(3):248–255. https://doi.org/10.1001/jama.2019.21187(Epub 2020/01/22, PubMed PMID: 31961420; PubMed Central PMCID: PMC6990678)
Feyz L, Theuns DA, Bhagwandien R, Strachinaru M, Kardys I, Van Mieghem NM et al (2019) Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin Res Cardiol 108(6):634–642. https://doi.org/10.1007/s00392-018-1391-3(Epub 2018/11/11, PubMed PMID: 30413869; PubMed Central PMCID: PMC6529371)
Romanov A, Pokushalov E, Ponomarev D, Strelnikov A, Shabanov V, Losik D et al (2017) Pulmonary vein isolation with concomitant renal artery denervation is associated with reduction in both arterial blood pressure and atrial fibrillation burden: data from implantable cardiac monitor. Cardiovasc Ther. https://doi.org/10.1111/1755-5922.12264(Epub 2017/04/20, PubMed PMID: 28423234)
Ukena C, Seidel T, Rizas K, Scarsi D, Millenaar D, Ewen S et al (2020) Effects of renal denervation on 24-h heart rate and heart rate variability in resistant hypertension. Clin Res Cardiol 109(5):581–588. https://doi.org/10.1007/s00392-019-01543-6(Epub 2019/09/25, PubMed PMID: 31555986)
Qiu M, Shan Q, Chen C, Geng J, Guo J, Zhou X et al (2016) Renal sympathetic denervation improves rate control in patients with symptomatic persistent atrial fibrillation and hypertension. Acta Cardiol 71(1):67–73. https://doi.org/10.2143/AC.71.1.3132100(Epub 2016/02/09, PubMed PMID: 26853256)
McLellan AJ, Schlaich MP, Taylor AJ, Prabhu S, Hering D, Hammond L et al (2015) Reverse cardiac remodeling after renal denervation: Atrial electrophysiologic and structural changes associated with blood pressure lowering. Heart Rhythm 12(5):982–990. https://doi.org/10.1016/j.hrthm.2015.01.039(Epub 2015/01/28, PubMed PMID: 25638699)
Tsioufis C, Papademetriou V, Tsiachris D, Dimitriadis K, Kasiakogias A, Kordalis A et al (2014) Drug-resistant hypertensive patients responding to multielectrode renal denervation exhibit improved heart rate dynamics and reduced arrhythmia burden. J Hum Hypertens 28(10):587–593. https://doi.org/10.1038/jhh.2014.14(Epub 2014/03/13, PubMed PMID: 24621623)
Jiang Z, Zhou X, Chen C, Wang Y, Fang P, Geng J et al (2018) Renal denervation for ventricular arrhythmia in patients with implantable cardioverter defibrillators. Int Heart J 59(2):328–332. https://doi.org/10.1536/ihj.17-129(Epub 2018/02/23, PubMed PMID: 29479013)
Evranos B, Canpolat U, Kocyigit D, Coteli C, Yorgun H, Aytemir K (2016) Role of adjuvant renal sympathetic denervation in the treatment of ventricular arrhythmias. Am J Cardiol 118(8):1207–1210. https://doi.org/10.1016/j.amjcard.2016.07.036(Epub 2016/07/29, PubMed PMID: 27600464)
Ukena C, Mahfoud F, Ewen S, Bollmann A, Hindricks G, Hoffmann BA et al (2016) Renal denervation for treatment of ventricular arrhythmias: data from an International Multicenter Registry. Clin Res Cardiol 105(10):873–879. https://doi.org/10.1007/s00392-016-1012-y(Epub 2016/06/30, PubMed PMID: 27364940)
Armaganijan LV, Staico R, Moreira DA, Lopes RD, Medeiros PT, Habib R et al (2015) 6-Month outcomes in patients with implantable cardioverter-defibrillators undergoing renal sympathetic denervation for the treatment of refractory ventricular arrhythmias. JACC Cardiovasc Interv 8(7):984–990. https://doi.org/10.1016/j.jcin.2015.03.012 (PubMed PMID: 26088516)
Schirmer SH, Sayed MM, Reil JC, Lavall D, Ukena C, Linz D et al (2015) Atrial remodeling following catheter-based renal denervation occurs in a blood pressure- and heart rate-independent manner. JACC Cardiovasc Interv 8(7):972–980. https://doi.org/10.1016/j.jcin.2015.02.014(Epub 2015/05/20, PubMed PMID: 26003031)
Ukena C, Mahfoud F, Spies A, Kindermann I, Linz D, Cremers B et al (2013) Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol 167(6):2846–2851. https://doi.org/10.1016/j.ijcard.2012.07.027(Epub 2012/08/20, PubMed PMID: 22917547)
Simantirakis EN, Papakonstantinou PE, Chlouverakis GI, Kanoupakis EM, Mavrakis HE, Kallergis EM et al (2017) Asymptomatic versus symptomatic episodes in patients with paroxysmal atrial fibrillation via long-term monitoring with implantable loop recorders. Int J Cardiol 231:125–130. https://doi.org/10.1016/j.ijcard.2016.12.025(Epub 20161221, PubMed PMID: 28041713)
Simantirakis EN, Papakonstantinou PE, Kanoupakis E, Chlouverakis GI, Tzeis S, Vardas PE (2018) Recurrence rate of atrial fibrillation after the first clinical episode: a prospective evaluation using continuous cardiac rhythm monitoring. Clin Cardiol 41(5):594–600. https://doi.org/10.1002/clc.22904(Epub 20180514, PubMed PMID: 29761516; PubMed Central PMCID: PMC6489953)
Ukena C, Mahfoud F, Linz D, Böhm M, Neuberger HR (2013) Potential role of renal sympathetic denervation for the treatment of cardiac arrhythmias. EuroIntervention 9(Suppl R):R110–R116. https://doi.org/10.4244/EIJV9SRA19(PubMed PMID: 23732141)
Kannel WB, Wolf PA, Benjamin EJ, Levy D (1998) Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 82(8A):2N-9N. https://doi.org/10.1016/s0002-9149(98)00583-9 (PubMed PMID: 9809895)
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP et al (2006) Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114(2):119–125. https://doi.org/10.1161/CIRCULATIONAHA.105.595140(Epub 2006/07/03, PubMed PMID: 16818816)
Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE (2010) Prevention of atrial fibrillation by renin–angiotensin system inhibition a meta-analysis. J Am Coll Cardiol 55(21):2299–2307. https://doi.org/10.1016/j.jacc.2010.01.043 (PubMed PMID: 20488299)
Wolf M, Ewen S, Mahfoud F, Böhm M (2018) Hypertension: history and development of established and novel treatments. Clin Res Cardiol 107(Suppl 2):16–29. https://doi.org/10.1007/s00392-018-1299-y(Epub 2018/06/27, PubMed PMID: 29951800)
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M (2008) Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 336(7653):1114–1117. https://doi.org/10.1136/bmj.39553.670231.25(Epub 2008/05/14, PubMed PMID: 18480115; PubMed Central PMCID: PMC2386633)
Lobo MD, Sharp ASP, Kapil V, Davies J, de Belder MA, Cleveland T et al (2019) Joint UK societies’ 2019 consensus statement on renal denervation. Heart 105(19):1456–1463. https://doi.org/10.1136/heartjnl-2019-315098(Epub 2019/07/10, PubMed PMID: 31292190; PubMed Central PMCID: PMC6817707)
Böhm M, Mahfoud F, Ukena C, Bauer A, Fleck E, Hoppe UC et al (2013) Rationale and design of a large registry on renal denervation: the Global SYMPLICITY registry. EuroIntervention 9(4):484–492. https://doi.org/10.4244/EIJV9I4A78 (PubMed PMID: 23965354)
Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA et al (2012) Renal denervation in moderate to severe CKD. J Am Soc Nephrol 23(7):1250–1257. https://doi.org/10.1681/ASN.2011111062(Epub 2012/05/17, PubMed PMID: 22595301; PubMed Central PMCID: PMC3380649)
Prasad B, Berry W, Goyal K, Dehghani P, Townsend RR (2019) Central blood pressure and pulse wave velocity changes post renal denervation in patients with stages 3 and 4 chronic kidney disease: the Regina RDN Study. Can J Kidney Health Dis 6:2054358119828388. https://doi.org/10.1177/2054358119828388(Epub 2019/02/13, PubMed PMID: 30792873; PubMed Central PMCID: PMC6376516)
Fukuta H, Goto T, Wakami K, Ohte N (2017) Effects of catheter-based renal denervation on heart failure with reduced ejection fraction: a systematic review and meta-analysis. Heart Fail Rev 22(6):657–664. https://doi.org/10.1007/s10741-017-9629-0 (PubMed PMID: 28646466)
Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N (2020) Effects of catheter-based renal denervation on heart failure with reduced ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. https://doi.org/10.1007/s10741-020-09974-4(Epub 2020/05/11, PubMed PMID: 32394227)
Ukena C, Becker N, Pavlicek V, Millenaar D, Ewen S, Linz D et al (2020) Catheter-based renal denervation as adjunct to pulmonary vein isolation for treatment of atrial fibrillation: a systematic review and meta-analysis. J Hypertens 38(5):783–790. https://doi.org/10.1097/HJH.0000000000002335 (PubMed PMID: 32238783)
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest (include appropriate disclosures)
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nantha Kumar, N., Nyatsuro, K., Ahmad, S. et al. Systematic review of renal denervation for the management of cardiac arrhythmias. Clin Res Cardiol 111, 971–993 (2022). https://doi.org/10.1007/s00392-021-01950-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01950-8