Abstract
Diabetic patients frequently develop heart failure with preserved (HFpEF) or mid-range (HFmEF) cardiac ejection fractions. This condition may be secondary to diabetic cardiomyopathy or one of several relevant comorbidities, mainly hypertension. Several mechanisms link diabetes to HFpEF or HFmEF. Among these, non-enzymatic glycation of interstitial proteins, lipotoxicity, and endothelial dysfunction may promote structural damage and ultimate lead to heart failure. Findings from several large-scale trials indicated that treatment with sodium/glucose cotransporter 2 inhibitors (SGLT2-iss) resulted in significant improvements in cardiovascular outcomes in diabetic patients with high cardiovascular risk. However, there is currently some evidence that suggests a clinical advantage of using SGLT2-iss specifically in cases of HFpEF or HFmEF. Preclinical and clinical studies revealed that SGLT2-iss treatment results in a reduction in left ventricular mass and improved diastolic function. While some of the beneficial effects of SGLT2-iss have already been characterized (e.g., increased natriuresis and diuresis as well as reduced blood pressure, plasma volume, and arterial stiffness, and nephron-protective activities), there is increasing evidence suggesting that SGLT2-iss may have direct actions on the heart. These findings include SGLT2-iss-mediated reductions in the expression of hypertrophic foetal genes and diastolic myofilaments stiffness, increases in global phosphorylation of myofilament regulatory proteins (in HFpEF), inhibition of cardiac late sodium channel current and Na+/H+ exchanger activity, metabolic shifts, and effects on calcium cycling. Preliminary data from previously published studies suggest that SGLT2-iss could be useful for the treatment of HFpEF and HFmEF. Several large ongoing trials, including DELIVER AND EMPEROR -preserved have been designed to evalute the efficacy of SGLT2-iss in improving clinical outcomes in patients diagnosed with HFpEF. The goal of this manuscript is to review the use of SGLT2-iss inhibitors for HFpEF or HFmEF associated with diabetes.
Similar content being viewed by others
Introduction
There is a strong relationship between diabetes and heart failure (HF). Diabetes has been associated with an increased risk of developing HF. Likewise, diabetic patients have an overall poorer prognosis, with higher cumulative hospitalizations, 1-year mortality, and 1-year rehospitalization rates for HF, even after adjustments for multiple clinical risk factors have been made [1, 2]. Guidelines published in 2016 by the European Society of Cardiology [3] recommended a definition of HF that was based on specified cut-offs of cardiac ejection fractions (EFs) and identified three specific HF phenotypes. These phenotypes include (1) HF with reduced ejection fraction (HFrEF), which includes patients with an EF ≤ 40%, and (2) HF with mid-range EF (HFmEF) in patients with an EF of 40–49%, and HF with preserved EF (HFpEF) for those with an EF ≥ 50%. The diagnosis of HFpEF or HFmEF also includes elevated levels of natriuretic peptides and evidence of structural heart disease or diastolic dysfunction. Even if the HF classification based on ejection fraction has been proven to be clinically useful, there are some criticisms that should be addressed.
EF is not a reliable measure of left ventricle contractility, because it depends largely on the ventricular loading. A reduction of left ventricular contractility, without left ventricular enlargement, could be associated to normal value of EF.
The classification of patients as HFmEF may be the result of the variability of the measure of EF.
In fact, there is a significant inter and intra observer variability when EF is measured by echocardiography, so the allocation of a patient, based on a single EF determination, especially for values near to the cut off points, is questionable.
To overcome the limitation of an EF-based classification of heart failure, a universal, comprehensive, classification of heart failure has been recently proposed [4]
The recent availability and clinical use of sodium/glucose cotransporter 2 inhibitors (SGLT2-iss) have advanced the field and provided new treatment modalities for both type 2 diabetes and HF. SGLT2-iss are oral antidiabetic drugs that induce the secretion of glucose into the urine by decreasing its reabsorption at the proximal convoluted tubule of the nephron. In large-scale trials, SGLT2-iss significantly improved cardiovascular (CV) outcomes in diabetic patients with high CV risk. This result was mainly driven by the reduced number of hospitalizations for heart failure (HHFs) [5–10]. In the EMPEROR-reduced and DAPA-HF trials, the addition of SGLT2-iss to standard therapy resulted in a reduction of CV death and events associated with HF in patients with HFrEF regardless of the diagnosis of diabetes [11, 12]. More recently, Bhatt and colleagues [13] reported that administration of the SGLT2-iss, sotagliflozin, resulted in reduced rates of hospitalization and urgent visits for symptoms related to HF as well as fewer deaths due to CV-related causes among diabetics with a recent history of worsening HF. At this time, the SGLT2-iss canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin are recommended for the prevention of HHFs in patients with Type 2 diabetes mellitus (T2DM) with established or at high risk for CV disease. Dapagliflozin and empagliflozin are recommended to reduce the risk of HHF and CV-associated deaths in patients with HFrEF in patients both with and without T2DM [14]
In this manuscript, we review the background and the scientific evidence underlying the use of SGLT2-iss in diabetic patients with HFpEF and HFmEF.
Diabetes and HFpEF/HFmEF
The disease phenotype HFpEF is defined by clinical symptoms that include HF, EF ≥ 50%, and echocardiographic evidence of diastolic dysfunction [15]. Nearly 30–40% of patients with HFpEF are also diabetic [16]. The principal biological findings associated with HFpEF include systemic inflammation, myocardial fibrosis, and vascular stiffness.
Impaired diastolic function coupled with aortic stiffness results in an arterial-ventricular mismatch that leads to increased left ventricular filling pressure. Signs and symptoms of vascular congestion emerge as a clinical consequence of this mismatch. Renal dysfunction frequently complicates this clinical scenario.
HFmEF is a heterogeneous condition and does not represent a stable phenotype. It is currently a “grey area” and is not recognized by some guidelines. HFmEF may be a transitional status and may represent either an improvement of HFrEF or a deterioration of HFpEF. While some of the older, pivotal trials addressing the complications of HF used a cut-off of 45% for left ventricular ejection fraction (LVEF), and thus included HFmEF with the more severe cases, more recent trials studied patients with more severe reductions only (i.e., EF < 30–35%). In clinical practice, patients with HFmEF are more often treated as though they were among those with HFrEF, most notably in cases with a history of partially recovered EF.
Clinical characteristics of diabetic patients with HFpEF
Among patients with HFpEF, diabetic subjects are overall younger, more obese, and more frequently male. These patients also have a higher prevalence of comorbidities (hypertension and renal dysfunction as well as pulmonary and vascular disease) and diminished exercise capacity [17]. Diabetic patients with HFpEF also exhibited more extensive cardiac remodelling, with more marked left ventricular hypertrophy with higher filling pressures, Doppler E-wave velocities, and E/e′ ratios compared to non-diabetic patients with this condition [17, 18]. Therefore, patients with diabetes may be more prone to volume retention, with increased peripheral and pulmonary congestion, higher natriuretic peptide concentrations, and higher rates of diuretic use [19]. These patients could derive substantial benefit from drug treatment that resulted in reduced plasma volume. Eventually, diabetic HFpEF patients developed a worse quality of life, with increased rates of hospitalization and CV-associated mortality compared to non-diabetic patients with HFpEF [17–19]. In the CHARM trial (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity), diabetic patients with an EF > 40% (including those with HFmEF and HFpEF) exhibited an adjusted twofold increase in CV disease or HHF, with an 80% increase in the risk of mortality for all-causes in comparison with non diabetic patients [20].
Several potential mechanisms link diabetes to HFpEF. Diabetic subjects often suffer from obesity, coronary disease, and kidney dysfunction, which are all conditions that promote HF. However, diabetic cardiomyopathy can develop even in the absence of other known risk factors for HF [21]. Left ventricular hypertrophy and diastolic impairment can develop in diabetic HFpEF patients even in the absence of pressure overload [22]. Rubler [23] was the first to identify and characterize diabetic cardiomyopathy in 1972. He identified structural cardiac abnormalities in a postmortem study of four diabetic patients who presented with no evidence of any other risk factors associated with HF. In 1974, data from the Framingham Heart study confirmed the role of diabetes in HF [24].
In 2001, the first classification based on a staging system for HF was proposed [25]. This staging system described the progressive course of the disease and included (1) stage A, which recognized the risk of developing HF; (2) stage B, the development of structural cardiac abnormalities without signs and symptoms of HF; (3) stage C, the presence of signs and symptoms of HF; and (4) stage D, representing end-stage HF. This staged classification underlines the importance of early identification of subjects at risk of developing HF (stage A). The use of appropriate therapeutic strategies in this patient cohort might limit the progression of the disease and may even prevent the development of end-stage HF.
Diabetes mellitus is a risk factor for the development of HFpEF among patients diagnosed at stage A and diabetic cardiomyopathy with diastolic dysfunction among those at stage B. The earliest stages of diabetic cardiomyopathy include long asymptomatic periods during which remodelling of the left ventricle takes place. During this phase, myocyte hypertrophy and increased stiffness develop along with increased myocardial fibrosis [26, 27]. As the heart remodelling process advances, diastolic dysfunction will develop together with the signs and symptoms of HFpEF. Further progression of this disease can lead to systolic dysfunction, a dilated left ventricle, a reduced ejection period, and an increase in filling pressures that are among clinical manifestations of HFrEF.
From diabetes to HFpEF
Several potential mechanisms have been identified that link diabetes to HFpEF.
Derangement in nutrient signalling
A nutrient excess signalling can been observed in diabetic cells; this redundant signal suppress autophagy, the main homeostatic process that, by the clearance of damaged mitochondria and peroxisomes, mitigates oxidative stress in diabetic cardiomyocytes [28].
Increased activity of sodium-hydrogen exchanger (NHE)
Increased intracellular sodium concentration has been associated to cardiac and kidney damage in diabetes. Hyperinsulinemia and hyperglycaemia may stimulate the expression of NHE in the heart and in the kidney. The increase of intracellular sodium, due to NHE activation, is associated to cardiac hypertrophy and fibrosis, glomerular hyperfiltration, and mesangial proliferation [29, 30].
Epicardial adipose tissue. Expansion of epicardial adipose tissue is another mechanism linking diabetes to heart failure.
Epicardial adipose tissue produces proinflamtory adipocytokine that causes cardiac inflammation, microcirculatory dysfunction, and fibrosis [31].
Protein glycation
The non-enzymatic glycation of interstitial proteins which are detected at increased levels secondary to sustained hyperglycaemia promote crosslinking between proteins and the production of advanced glycation end products (AGEs). Accumulation of AGEs can promote CV complications associated with diabetes via the modification of both extracellular and intracellular proteins.
Proteins found in the extracellular matrix, including collagen, myelin, tubulin, plasminogen activator 1, and fibrinogen, can all be modified to become AGEs [16, 32]. Collagens crosslinking results in increased left ventricular stiffness and ultimately to diastolic dysfunction [16, 33]. Increases in extracellular volume (ECV) observed in patients with T2DM confirm the role of extracellular matrix changes in this setting [34].
At the intracellular level, AGEs in cardiomyocytes create crosslinks between the domains of both the ryanodine receptors and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), leading to alterations in calcium trafficking [35, 36].
Increase deposition of free fatty acid
Lipotoxicity and oxidative stress are also among the pathological mechanisms that promote the development of HFpEF in patients with diabetes. Heart tissue from diabetic patients generates energy mainly via the oxidation of fatty acids. As a result, the deposition of free fatty acids and non-esterified fatty acids increases. Adverse effects include the production of ceramide, which promotes cellular apoptosis [37, 38].
Endothelial dysfunction
A pivotal derangement found in both diabetes and HFpEF is a derangement of endothelial function. Thus, ED plays a leading role in the crosstalk between these two conditions. ED is characterized by reduced production and/or availability of nitric oxide (NO), which results in numerous deleterious consequences, including impaired vasodilation, increased vasoconstriction, arterial stiffness, and atherogenesis. Moreover, in diabetic patients, ED has been associated with an imbalance of vascular growth factors [39] and an increase in reactive oxygen species (ROS). These factors contribute to chronic renin–angiotensin–aldosterone system (RAAS) activation, tissue fibrosis, and renal disease. The decline in glomerular filtration, together with heart disease, promotes the downhill trajectory of this disease.
Early diagnosis of this condition is fundamental. It will be critical to have some means to detect both structural and functional cardiac anomalies even before the onset of the signs and symptoms of HF. This can be achieved by non-invasive methods such as echocardiography [31]. Two-dimensional (2D)-speckle-tracking echocardiography (STE) has recently been validated as a method that can be used to identify an abnormal global longitudinal strain in diabetic patients with normal EF, a condition that has been found in 30–50% of cases. This technique could be used to identify early indicators of cardiac dysfunction and early stages of cardiomyopathy in diabetic patients [40].
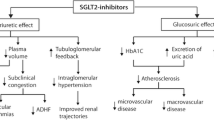
Mechanisms linking diabetes to HFpEF are reported in Fig. 1.
How diabetic patients with HFpEF/HFmEF might benefit from SGL2-is
The beneficial effects of SGLT2-iss on both cardiac structure and function suggest that they may have therapeutic utility in patients with diabetes and HFpEF. Patients in this cohort might respond to these drugs and achieve results that are similar to those observed in patients with HFrEF.
Effects of SGLT2-iss on cardiac structure and function
Hammoudi and colleagues [41] evaluated the impact of SGLT2-iss therapy in a genetic model of T2DM. Among their findings, they reported that T2DM obese mice treated with empagliflozin exhibited improved diastolic function compared to untreated controls as determined by mitral flow pattern using 2D-echocardiography. These findings were confirmed by hemodynamic measurements. However, treatment had no impact on inotropic responses.
SGLT2-iss therapy has also been examined in human studies. The EMPA-HEART Cardiolink-6 randomized clinical trial [42] was a double-blind randomized placebo-controlled trial in which individuals with T2DM and coronary heart disease were randomized to receive empagliflozin (10 mg/day) or placebo. Empagliflozin was associated with a significant reduction in left ventricular mass indexed to body surface area (LVMi) as measured by cardiac magnetic resonance (CMR) after 6 months of treatment. Moreover, the empagliflozin treatment group exhibited significant reductions in both systolic and diastolic blood pressure and elevated hematocrits, the latter likely to be a secondary response to reductions in plasma volume. The reduction in LVMi may be the result of decreased cardiomyocyte mass or a reduction in interstitial fluid levels.
A sub-study of the EMPA-HEART Cardiolink-6 trial that used CMR for tissue characterization demonstrated that treatment with empagliflozin resulted in a significant reduction in ECV, which has been identified as a marker of extracellular cardiac remodelling [43].
Mechanisms underlying the benefits of SGLT2-iss therapy
The mechanisms underlying the beneficial outcomes observed in response to SGLT2-iss therapy, most notably those related to HF, are likely to be multifactorial in nature. An analysis of data from the CANVAS program revealed that administration of canagliflozin resulted in the modification of 14 different potentially beneficial mediators, with the largest impact observed on erythrocyte concentration, hemoglobin levels, and serum urate concentration [44].
Both indirect (non-cardiac) and direct (cardiac) mechanisms may serve to explain the beneficial effects of SGLT2-iss and their capacity to modulate cardiac structural abnormalities.
The main mechanisms of the action of SGLT2-iss are those promoting the enhanced renal excretion of glucose. This serves to reduce hyperglycemia and to increase natriuresis and diuresis. These effects could promote organ decongestion and reductions in ECV, thereby improving tissue perfusion and reducing the hypertrophic stimulus associated with tissue hypoxia. Moreover, natriuresis and osmotic diuresis will serve to reduce both preload and afterload and will result in reductions in blood pressure, plasma volume, and arterial stiffness. Of interest, reductions in blood pressure were reported as part of the EMPA-HEART Cardiolink-6 study. However, changes in the LVMi were not associated with changes in the 24-h ambulatory pressure. This result suggests that other mechanisms may be involved in promoting reductions in LVMi observed in patients treated with empagliflozin [42].
Improved glycaemic control alone may also explain the observed reduction in HF outcomes associated with SGLT2-iss therapy. Reductions in prolonged hyperglycaemia will result in reduced rates of generation of ROS and AGEs. Moreover, treatment with SGLT2-iss limits the deterioration of the glomerular filtration rate in T2DM and limits progression to macroalbuminuria [45]. Given that renal disease is a major comorbidity associated with T2DM and is associated with a poor prognosis of patients with HF, SGLT2-iss-mediated nephron protection may be the major source of its protective effects.
In addition to the recognized non-cardiac effects of SGL2-is, there is increasing interest in direct actions of this class of antidiabetic drug on cardiac tissue [46]. While SGLT2 is not highly expressed in cardiac tissue [47], the positive effects of SGLT2-iss, specifically, empagliflozin, on cardiac function have been observed experimentally in isolated hearts. Under these conditions, the impact of these drugs would be unrelated to hemodynamic changes or other non-cardiac factors [48]. In these studies, empagliflozin treatment resulted in the reduction of some markers of hypertrophy (i.e., expression of hypertrophic foetal genes) and had a beneficial effect on calcium cycling, which has been identified as a major determinant of diastolic function [41].
Interestingly, reduced diastolic dysfunction secondary to empagliflozin treatment was observed in isolated ventricular trabeculae from patients with systolic end-stage HF. This effect was not explained by altered Ca2+ homeostasis; in this study, empagliflozin did not modify the amplitude of systolic calcium transients or have any impact on its decay kinetics. Instead, empagliflozin treatment resulted in increased global phosphorylation of myofilament regulatory proteins in patients with HFpEF. This resulted in reduced myofilament stiffness in diastole and thus in a direct improvement of diastolic function [49]. Of note, this diastolic improvement was observed immediately after intravenous infusion of empagliflozin. Collectively, these findings suggest that the beneficial effects of SGLT2-iss may be independent of diabetic status.
The direct effect of SGLT2-iss on cardiac tissue is also suggested by results of experiments that feature reductions in the development of HF in non-diabetic mice. These responses were associated with inhibition of the nuclear binding domain-like receptor 3 inflammasome nucleosome (NLRP3) [50] in an ex vivo model of cardiac injury in the absence of hemodynamic or metabolic alterations.
SGLT2-iss can target some pathophysiological pathways of diabetic cardiomyopathy, improving the prognosis of diabetic patients with diabetes, beyond what is achieved when glucose control is obtained by other means.
The loss of glucose (and calories) by inhibiting SGLT2 may increase autophagy, inducing up-regulation of nutrient deprivation signals. Enhanced autophagy has been associates to several beneficial cardiac effects [28].
Increase of intracellular sodium, induced by enhanced expression of NHE, has a pivotal role in diabetic cardiomyopathy.
SGLT2-iss may also reduce the detrimental impact of HF on sodium homeostasis via its capacity to inhibit currents through the late sodium channel [51] that promote both cardiac failure and arrhythmias [52] and also via its impact on Na+/H+ exchange activity (which is increased in HFpEF) [53, 54].
SGLT2-iss have a positive effect of on human epicardia adipose tissue. They reduce the glucose uptake of mature adipocytes and the secretion of chemokines [55]
Some researchers hypothesized that SGLT2-iss can modulate changes in substrate utilization in the heart. This would represent yet another cardioprotective mechanism attributed to the actions of SGLT2-iss. Administration of these drugs induces persistent hyperketonemia; in these cases, β-hydroxybutyrate is freely available for energy production in the heart, and ketones are oxidized in preference to fatty acids. This metabolic shift improves work efficiency at the mitochondrial level and reduces oxidative stress [56].
Possible mechanisms of benefit in pts with T2DM and HFpEF/HFmEF are in Table 1.
Clinical evidence of the benefits of SGLT2-iss therapy in patients with HFpEF and HFmEF
The Swedish HF registry includes data from 6805 patients with HF and T2DM. Among these are patients with reduced, mid-range, and preserved EF (48%, 24.1%, and 27.9%, respectively). Analysis of these data led to the conclusion that the use of SGLT2-iss was associated with lower morbidity (hospitalizations for CV causes and HF) and reduced mortality [57]. The overall cohort event rates for CV-associated death or first HF hospitalization were 193 versus 328 per 1000 patient-years among those treated with SGLT2-iss compared to the no-SGLT2-iss group, respectively, which corresponded to an unadjusted hazard ratio (HR) of 0.53 (95% CI 0.40–0.70). The authors of this study reported no interactions between EF and the association of SGLT2-iss with CV-associated deaths and HHFs. A similar association of SGLT2-iss use with outcome was observed across the entire spectrum of EFs.
Empagliflozin did not increase exercise capacity in patients with HFpEF among participants in the EMPERIAL trial (Effect of EMPagliflozin on ExeRcise ability and HF symptoms). However, the short duration of the trial (12 weeks) might explain the lack of efficacy with respect to this functional outcome [58].
To date, there are no published clinical trials that were designed to evaluate the efficacy of SGLT2-iss in reducing the extent of hard clinical endpoints in patients with T2DM and HFpEF or HFmEF. However, we can extrapolate from the results of several previously published large trials that enrolled a significant number of patients with HF that (in some cases) included data on LVEFs. For example, the CAVAS program [59] included 10,142 participants with T2DM at high risk for developing CV. The participants were randomized to receive either canagliflozin or a placebo. Among the results, participants with a history of HF experienced greater reductions in the risk of CV-associated deaths and HHFs compared to participants with no history of HF. However, LVEF data were not routinely collected at enrolment. Thus, it is not possible to distinguish between the results based on different LVEF classes.
Similarly, data from the CREDENCE trial demonstrated that treatment with canagliflozin reduced renal and CV events in patients with prior history of baseline HF (15% of the entire population), but not classified based on LVEF [60]. DECLARE-TIMI 58 [61] was the first trial involving SGLT2-iss that reported detailed information on LVEFs of participants with a history of HF. The trial compared outcomes in participants with T2DM with either multiple risk factors or established atherosclerotic CV disease that were treated with 10 mg per day empagliflozin or placebo. Among the 17,160 participants, 1987 (12%) presented with a history of HF. Of this subgroup, 3.9% presented with an EF < 45% and were classified as HFrEF. Another 7.7% were classified as HF without reduced HF; this group included 808 patients with EF ≥ 45% and 508 with no EF data available). Participants with HFmEF were included in both groups. In this study, dapagliflozin reduced the composite risk of CV-associated death or HHF to a larger extent in patients diagnosed with HFrEF (HR 0.62; 95% CI 0.45–0.86) than in those without reduced EF (HR 0.88; 95% CI 0.76–1.02), although no differences in HHF were observed in response to dapagliflozin treatment among those with HF that presented with or without a reduced EF (HR 0.64; 95% CI 0.43–0.95 and HR 0.76; 95% CI 0.623–0.92, respectively; p for interaction = 0.45). The HR for CV-associated death and HHF was 0.88 (95% CI 0.6–1.17) for participants who presented with established HFpEF, while HR for HHF was 0.7 (95% CI 0.50–1.04). Interestingly, the benefits of dapagliflozin for reducing the primary endpoint in HFrEF were observed early in the trial and were different for participants with HF but without reduced EF versus those without HF; the event curves for HHF diverged after 1 year. The beneficial mechanisms may differ based on the presence or absence of systolic dysfunction.
VERTIS CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial) was a randomized, double-blind, multicenter trial that targeted patients with T2DM and established atherosclerotic CV disease in which the effects of ertugliflozin with placebo on CV, renal, and metabolic outcomes were compared [9].
Study participants were randomly assigned (in a 1:1:1 ratio) to treatment protocols that included ertugliflozin 5 mg, ertugliflozin 15 mg, or matching placebo once daily. Among 8246 randomized participants, 1958 presented with a history of HF at baseline. LVEF data were available for 1485 (76%) patients; of these, 1007 (68%) presented with an EF > 45% and 478 (32%) with an EF ≤ 45%. Patients were followed for a median of 3.0 years. Overall, ertugliflozin reduced risk for first HHF (HR 0.70; 95% CI, 0.54–0.90). Ertugliflozin had a similar positive impact on participants with baseline HF (HR 0.63; 95% CI 0.44–0.90). In participants with HF, the risk reduction for a first HHF was similar among those with reduced EF (≤ 45%) versus preserved EF (> 45%) or cases in which the EF was not known [62].
SOLOIST-WHF [13] is the only trial published to date in which an SGLT2-iss (sotagliflozin) was used in a study limited to diabetic HF patients who presented with either reduced or preserved EFs. Patients were eligible for enrolment if they had been hospitalized for signs and symptoms of HF and had undergone treatment with an intravenous diuretic and were maintained on oral therapy at the time of inclusion. The trial design specifically targeted patients who had undergone recent treatment for clinically evident congestion. Note that Consensus, which demonstrated the efficacy of an angiotensin-converting enzyme (ACE) inhibitor in improving clinical outcomes in HF, included patients with New York Heart Association (NYHA) class IV disease, with signs of fluid retention, although no measurement of cardiac function was required [63]. In the Soloist trial, randomization was stratified according to LVEF (< 50% or ≥ 50%). Enrolled patients were randomized to receive 200 mg of sotagliflozin once daily or placebo, and treatment was initiated either before or three days after hospital discharge. The study enrolled 1222 participants, 21% of whom were diagnosed with HFpEF. The median estimated glomerular filtration rates (GFRs) were 49.2 and 50.5 ml/min/1.73 m2 of body surface area in the sotagliflozin and placebo treatment groups, respectively. After a median follow-up of 9 months, the frequency of the primary endpoint (a composite score of CV-associated deaths, hospitalizations, and urgent visits for HF) was significantly lower in the actively treated group (51.0 versus 76.3; HR 0.67; 95% CI 0.52–0.85). This outcome was driven primarily by the reduction in the number of hospitalizations and urgent visits for issues related to HF (HR 0.64; 95% CI 0.49–0.83). This result was observed consistently across specified subgroups, including those stratified based on EF. In participants with EF ≥ 50%, the reduction in the frequency of the primary endpoint was larger than among those with EF < 50%, albeit with a comparatively large CI. The HRs (CI) associated with these findings were 0.48 (0.27–0.86) and 0.72 (0.56–0.94) for subgroups with LVEF ≥ 50% and < 50%, respectively.
The SCORED trial [10] evaluated the efficacy of sotagliflozin in reducing the composite value representing the total deaths from CV-associated causes, HHFs, and urgent visits for HF in patients with T2DM, a high CV risk, and chronic kidney disease. Sotagliflozin was provided at 200 mg per day, with a dose increase to 400 mg per day in the absence of unacceptable side effects. Approximately 31% of participants enrolled in this study presented with a history of HF. Among this group, the prevalence of HFpEF, HFrEF, and HFmEF was 51%, 31%, and 18%, respectively. During a median follow-up period of 16 months, primary endpoint events were reported at frequencies of 5.6 and 7.5 events per 100 patient-years in the sotagliflozin and placebo groups, respectively (HR 0.74; 95% CI 0.63–0.88; p < 0.001). Moreover, among the participants who presented with a diagnosis of HF at enrolment, sotagliflozin significantly reduced the frequency of the primary endpoint compared to the placebo. The frequencies of primary endpoint events were 15.9 and 20.6 events per 100 patient-years in the sotagliflozin and placebo groups, respectively (HR 0.77; 95% CI 0.61–0.96). When patients were grouped according to EF, the HRs (CIs) for the reduction of primary endpoint rates for those in the HFpEF, HFrEF, or HFmEF groups were 0.72 (0.52–0.99), 0.95 (0.7–0.1.28), and 0.50 (0.32–0.77). While participants with HFmEF appeared to be the most likely to benefit from sotagliflozin therapy, this sub-analysis should be interpreted with caution, given the comparatively small size of the sample.
In a recent meta-analysis of six trials that featured the use of SGLT-is in patients presenting with HF, Singh and colleagues [64] identified a similar risk reduction in the composite value representing CV-associated deaths and HHFs in participants with HFpEF (HR 0.75; 95% CI 0.62–0.91) and HFrEF (HR 0.74; 95% CI 0.68–0.80).
The EMPEROR-preserved trial [65] is a multicenter, randomized, double-blind, parallel-group, placebo-controlled trial that was designed to evaluate the impact of empagliflozin on morbidity and mortality in patients with established HFpEF with or without T2DM. All participants included in the study presented with chronic heart failure (functional class II, III, or IV) for at least 3 months duration, an LVEF > 40% at most recent prior assessment, and no history of EFs ≤ 40% recorded. All participants must also have elevated levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP). Participants included in the study are randomized into groups that are treated with a placebo or empagliflozin (10 mg/day) in addition to their usual therapy for HF.
The primary endpoint of the EMPEROR-Preserved Trial is the time-to-first-event of the combined risk for adjudicated CV-associated death and HHF. Baseline characteristics of the study population have been published [66]. Nearly half of the enrolled participants carried a diagnosis of T2DM (49%) and chronic kidney disease as defined by an eGFR < 60 mL/min/1.73 m2 (50%). Twenty-three percent of the participants reported an HHF within the past 12 months. The mean LVEF among those in the study population is 54 ± 9%. Thirty-three percent of the participants presented with HFmEF with a baseline LVEF of 41–50%.
During a median follow-up of 26.3 months, a primary outcome end-point was observed in 415 of 2997 patients (13.8%) in the empagliflozin group and in 511 of 2991 patients in the placebo group (hazard ratio 0.79; 95% of confidence interval 0.69 to 0.90) [67].
These results were consistent in patients with diabetes. In patients with diabetes at baseline, a primary outcome event occurred in 239 of 1466 patients in the empagliflozin group and 291 of 1472 patients in the placebo group (hazard ratio 0.71; 95% of confidence interval 0.67 to 0.94).
Studies that explored the clinical effects of SGLT2-iss therapy in patients with HFpEF/HFmEF and T2DM are reported in Tables 2 and 3.
In summary, published evidence suggests that patients with T2DM and HFpEF or HFmEF might benefit from treatment with SGLT2-iss. This question needs to be addressed properly in appropriately designed clinical trials to reach a definitive understanding of this important clinical issue.
A main gap of knowledge in this field is the poor evidence for patients with HFmEF; in fact, this group of patients is often associated to the preserved ejection fraction group.
Next trials should separate patients with mildly reduced ejection fraction to evaluate the effects of SGLT2-iss in this subgroup.
However, in future trials, the limits of the heart failure classification based on ejection fraction should be overcome, and a more accurate profiling of study populations should be used.
Furthermore, the incremental advantage of SGLT2-iss in comparison with other antidiabetic drugs in improving cardiovascular outcome should be explored with correct study designs.
Eventually, even if HHF hospitalization is a universally considered a relevant outcome, future studies should be designed with the right statistical power to evaluate the effect of SGLT2-iss on the most important outcome: i.e. total mortality.
Ongoing studies
Many of the published trials that feature SGLT2-iss as treatment modalities for patients with diabetes and HFpEF/HFmEF aimed to identify their favourable biological mechanisms as well as their efficacy in improving functional capacity, quality of life, and clinical outcomes [68]. In many studies, patients with HFpEF/HFmEF are included together in one group.
DELIVER trial is a phase III international, multicenter, parallel-group, randomized, double-blind, placebo-controlled study designed to evaluate dapagliflozin (10 mg per day) versus placebo in patients with HFpEF. Inclusion criteria include an LVEF > 40% and evidence of structural heart disease (i.e., left ventricular hypertrophy or left atrial enlargement) documented before enrolment. Elevated NT-pro BNP levels are also required. In contrast to the EMPEROR trial, the DELIVER trial enrolled and randomized both ambulatory and hospitalized patients. The primary outcome measure of the DELIVER trial is the time to the first occurrence of CV-associated death, HHF, or urgent visit for HF [69].
Conclusion
SGLT2-iss reduce the risk of CV events and HHF in patients diagnosed with T2DM. Moreover, SGLT2-iss treatment results in improved clinical outcomes in patients with HFrEF regardless of their diabetes status. To date, there are no proven specific therapies that result in improved prognosis among patients with HFpEF. Favourable SGLT2-iss-mediated mechanisms might provide benefit to this otherwise difficult-to-treat group of patients. An analysis of published trials, notably those in which LVEF data are available, provides encouraging clues. However, additionally, more definitive insight into this issue awaits the conclusion of the ongoing DELIVER and EMPEROR-preserved clinical trials.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Kenny HC, Abel ED (2019) Heart failure in type 2 diabetes mellitus. Circ Res 124:121–141. https://doi.org/10.1161/CIRCRESAHA.118.311371
Targher G, Dauriz M, Laroche C, Temporelli PL, Hassanein M, Seferovic et al (2017) ESC-HFA HF Long-Term Registry investigators. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail 19:54–65. https://doi.org/10.1002/ejhf.679
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 37:2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Bozkurt B, Coats A, Tsutsui H (2021) Universal definition and classification of heart failure. J Card Fail 7:S1071–9164(21)00050–6. https://doi.org/10.1016/j.cardfail.2021.01.022
Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A et al (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 37:1526–1534. https://doi.org/10.1093/eurheartj/ehv728
Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE et al (2019) Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 139:1384–1395. https://doi.org/10.1161/CIRCULATIONAHA.118.037778
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, CANVAS Program Collaborative Group et al (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657. https://doi.org/10.1056/NEJMoa1611925
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A et al (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380:347–357. https://doi.org/10.1056/NEJMoa1812389
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U et al (2020) Cardiovascular outcomes with Ertugliflozin in type 2 diabetes. N Engl J Med 383:1425–1435. https://doi.org/10.1056/NEJMoa2004967
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK et al (2021) Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 384:129–139. https://doi.org/10.1056/NEJMoa2030186
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383:1413–1424. https://doi.org/10.1056/NEJMoa2022190
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK et al (2021) Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384:117–128. https://doi.org/10.1056/NEJMoa2030183
Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T et al (2020) Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 22:1495–1503. https://doi.org/10.1002/ejhf.1954
Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE et al (2007) Eur Heart J 28:2539–2550. https://doi.org/10.1093/eurheartj/ehm037
Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA (2018) Heart failure with preserved ejection fraction in diabetes: mechanisms and management. Can J Cardiol 34:632–643. https://doi.org/10.1016/j.cjca.2018.02.026
Lindman BR, Dávila-Román VG, Mann DL, McNulty S, Semigran MJ, Lewis GD et al (2014) Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 64:541–549. https://doi.org/10.1016/j.jacc.2014.05.030
Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S et al (2017) Circulation 135:724–735. https://doi.org/10.1161/CIRCULATIONAHA.116.024593
Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B (2010) Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol 105:373–377. https://doi.org/10.1016/j.amjcard.2009.09.04
MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB et al (2008) Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 29:1377–1385. https://doi.org/10.1093/eurheartj/ehn153
Lam CS (2015) Diabetic cardiomyopathy: an expression of stage B heart failure with preserved ejection fraction. Diab Vasc Dis Res 12:234–238. https://doi.org/10.1177/1479164115579006
van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K et al (2008) Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117:43–51. https://doi.org/10.1161/CIRCULATIONAHA.107.7285
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602. https://doi.org/10.1016/0002-9149(72)90595-4
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34:29–34. https://doi.org/10.1016/0002-9149(74)90089-7
Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS et al (2001) ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary circulation 104:2996–3007. https://doi.org/10.1161/hc4901.102568
Jia G, Hill MA, Sowers JR (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122:624–638. https://doi.org/10.1161/CIRCRESAHA.117.311586
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18:149–166. https://doi.org/10.1007/s10741-012-9313-3
Packer M (2021) Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail 9:535–549. https://doi.org/10.1016/j.jchf.2021.05.019
Anzawa R, Seki S, Nagoshi T, Taniguchi I, Feuvray D, Yoshimura M (2012) The role of Na+/H+ exchanger in Ca2+ overload and ischemic myocardial damage in hearts from type 2 diabetic db/db mice. Cardiovasc Diabetol 11(11):33. https://doi.org/10.1186/1475-2840-11-33.)
Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S (2008) Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res 103:891–899. https://doi.org/10.1161/CIRCRESAHA.108.175141
Packer M (2020) Epicardial adipose tissue inflammation can cause the distinctive pattern of cardiovascular disorders seen in psoriasis. Am J Med 133:267–272. https://doi.org/10.1016/j.amjmed.2019.08.027
Fukami K, Yamagishi S, Okuda S (2014) Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des 20:2395–2402. https://doi.org/10.2174/13816128113199990475
Hegab Z, Gibbons S, Neyses L, Mamas MA (2012) Role of advanced glycation end products in cardiovascular disease. World J Cardiol 4:90–102. https://doi.org/10.4330/wjc.v4.i4.90
Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS et al (2014) Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 35:657–664. https://doi.org/10.1093/eurheartj/eht193
Bidasee KR, Nallani K, Yu Y, Cocklin RR, Zhang Y, Wang M et al (2003) Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 52:1825–1836. https://doi.org/10.2337/diabetes.52.7.1825
Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, Besch HR Jr (2004) Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 53:463–473. https://doi.org/10.2337/diabetes.53.2.463
Poornima IG, Parikh P, Shannon RP (2006) Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98:596–605. https://doi.org/10.1161/01.RES.0000207406.94146.c2
Zhang DX, Fryer RM, Hsu AK, Zou AP, Gross GJ, Campbell WB, Li PL (2001) Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res Cardiol 96:267–274. https://doi.org/10.1007/s003950170057
Ricciardi CA, Gnudi L (2021) Vascular growth factors as potential new treatment in cardiorenal syndrome in diabetes. Eur J Clin Invest 3:e13579. https://doi.org/10.1111/eci.1357
Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V (2017) Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovasc Diabetol 16:130. https://doi.org/10.1186/s12933-017-0615-6
Hammoudi N, Jeong D, Singh R, Farhat A, Komajda M, Mayoux E et al (2017) Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes. Cardiovasc Drugs Ther 3:233–246. https://doi.org/10.1007/s10557-017-6734-1
Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H et al (2019) Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 140:1693–1702. https://doi.org/10.1161/CIRCULATIONAHA.119.042375
Mason T, Coelho-Filho OR, Verma S, Chowdhury B, Zuo F, Quan A et al (2021) Empagliflozin reduces myocardial extracellular volume in patients with type 2 diabetes and coronary artery disease. JACC Cardiovasc Imaging 14:1164–1173. https://doi.org/10.1016/j.jcmg.2020.10.017
Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW et al (2020) Mediators of the effects of Canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 8:57–66. https://doi.org/10.1016/j.jchf.2019.08.004
Williams DM, Nawaz A, Evans M (2020) Renal outcomes in type 2 diabetes: a review of cardiovascular and renal outcome trials. Diabetes Ther 11:369–386. https://doi.org/10.1007/s13300-019-00747-3
Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW et al (2018) Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol 9:1575. https://doi.org/10.3389/fphys.2018.0157
Di Franco A, Cantini G, Tani A, Coppini R, Zecchi-Orlandini S, Raimondi L et al (2017) Int J Cardiol 243:86–90. https://doi.org/10.1016/j.ijcard.2017.05.032
Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G et al (2017) Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci 2:347–354. https://doi.org/10.1016/j.jacbts.2017.07.003
Pabel S, Wagner S, Bollenberg H, Bengel P, Kovács Á, Schach C et al (2018) Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail 20:1690–1700. https://doi.org/10.1002/ejhf.1328
Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM et al (2020) Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail 13:e006277. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006277
Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, Barr A et al (2021) Circulation 143:2188–2204. https://doi.org/10.1161/CIRCULATIONAHA.121.053350
Makielski JC (2016) Trends Cardiovasc Med 26:115–122. https://doi.org/10.1016/j.tcm.2015.05.006
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A et al (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61:722–726. https://doi.org/10.1007/s00125-017-4509-7
Trum M, Riechel J, Lebek S, Pabel S, Sossalla ST, Hirt S (2020) ESC Heart Fail 7:4429–4437. https://doi.org/10.1002/ehf2.13024
Díaz-Rodríguez E, Agra RM, Fernández ÁL, Adrio B, García-Caballero T et al (2018) S.Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res 114:336–346. https://doi.org/10.1093/cvr/cvx186
Ferrannini E, Mark M, Mayoux E (2016) CV Protection in the EMPA-REG OUTCOME ttrial: a “thrifty substrate” hypothesis. Diabetes Care 39:1108–1114. https://doi.org/10.2337/dc16-0330
Becher PM, Schrage B, Ferrannini G, Benson L, Butler J, Carrero JJ et al (2021) Use of sodium-glucose co-transporter 2 inhibitors in patients with heart failure and type 2 diabetes mellitus: data from the Swedish Heart Failure Registry. Eur J Heart Fail 23:1012–1022. https://doi.org/10.1002/ejhf.2131
Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS et al (2021) Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J 42:700–710. https://doi.org/10.1093/eurheartj/ehaa943
Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D et al (2018) Circulation 138:458–468. https://doi.org/10.1161/CIRCULATIONAHA.118.034222
Sarraju A, Li J, Cannon CP, Chang TI, Agarwal R, Bakris G et al (2021) Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: Results from the CREDENCE trial. Am Heart 233:141–148. https://doi.org/10.1016/j.ahj.2020.12.008
Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM et al (2019) Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 139:2528–2536. https://doi.org/10.1161/CIRCULATIONAHA.119.040130
Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S et al (2020) Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation 142:2205–2215. https://doi.org/10.1161/CIRCULATIONAHA.120.05025
CONSENSUS Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 316:1429–1435. https://doi.org/10.1056/NEJM198706043162301
Singh AK, Singh R, Misra A (2021) Do SGLT-2 inhibitors exhibit similar cardiovascular benefit in patients with heart failure with reduced or preserved ejection fraction? J Diabetes 13:596–600. https://doi.org/10.1111/1753-0407.13182
Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J et al (2019) Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail 21:1279–1287. https://doi.org/10.1002/ejhf.1596
Anker SD, Butler J, Filippatos G, Shahzeb KM, Ferreira JP et al (2020) Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur J Heart Fail 22:2383–2392. https://doi.org/10.1002/ejhf.2064
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. Online ahead of print. https://doi.org/10.1056/NEJMoa2107038
https://clinicaltrials.gov/ct2/results?cond=heart+failure+preserved&term=sglt2&cntry=&state=&city=&dist=. Accessed 16 July 2021
Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN et al (2021) Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial Eur J Heart Fail May 29. Online ahead of print. https://doi.org/10.1002/ejhf.2249
Author information
Authors and Affiliations
Contributions
AP, CR, AlP, and DS contributed to the conception and design of the review. AP and CR wrote sections of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Passantino, A., Rizzo, C., Scrutinio, D. et al. Diabetes and SGLT2-iss inhibitors in patients with heart failure with preserved or mid-range left ventricular ejection fractions. Heart Fail Rev 28, 683–695 (2023). https://doi.org/10.1007/s10741-021-10186-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10186-7