Abstract
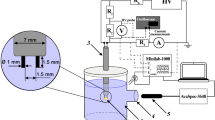
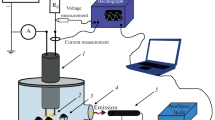
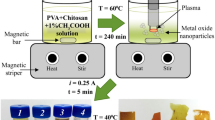
The novel three-electrode underwater pulse discharge excited by two independent high-voltage sources was used for the synthesis of W-Mo mixed oxide nanoparticles for the first time. The spectral and optical characteristics of the discharge were studied. It was found that the composition of the formed mixed oxides particles can be controlled by the discharge current between the anodes—general cathode pair. The chemical composition and morphology of obtained particles were studied by using X-ray diffraction spectroscopy and electron microscopy. The dynamic light scattering was used to measure the average particle diameter and zeta-potential. The photocatalytic performance of mixed oxides nanoparticles was estimated through the degradation of Rhodamine B under visible light irradiation conditions. It was found that the composition of the nanoparticles affects the photocatalytic properties.











Similar content being viewed by others
References
Kostov KG, Nishime TMC, Castro AHR, Toth A, Hein LRO (2014) Surface modification of polymeric materials by cold atmospheric plasma jet. Appl Surf Sci 314:367
Akram M, Jansen KMB, Ernst LJ, Bhowmik S (2011) Atmospheric pressure plasma surface modification of titanium for high temperature adhesive bonding. Int J Adhes Adhes 31:598
Abenojar J, Martinez MA, Encinas N, Velasco F (2013) Modification of glass surfaces adhesion properties by atmospheric pressure plasma torch. Int J Adhes Adhes 44:1
Hijosa-Valsero M, Molina R, Schikora H, Muller M, Bayona JM (2013) Removal of priority pollutants from water by means of dielectric barrier discharge atmospheric plasma. J Hazard Mater 262:664
Kim HS, Cho YI, Hwang IH, Lee DH, Cho DJ, Rabinovich A, Fridman A (2013) Use of plasma gliding arc discharges on the inactivation of E. Coli in Water Sep Purif Technol 120:423
Yamatake A, Fletcher J, Yasuoka K, Ishii S (2006) Water treatment by fast oxygen radical flow with Dc-driven microhollow cathode discharge. IEEE Trans Plasma Sci 34:1375
Yang Y, Cho YI, Fridman A (2012) Plasma discharge in liquid: water treatment and applications. CRC Press, New York
Webb MR, Andrade FJ, Gamez G (2005) High-throughput elemental analysis of small aqueous samples by emission spectrometry with a compact, atmospheric-pressure solution-cathode glow discharge. J Anal At Spectrom 20:1218
Mezei P, Cserfalvi T (2007) Electrolyte cathode atmospheric glow discharges for direct solution analysis. Appl Spectr Rev 42:573
Bencs L, Laczai N, Mezei P, Cserfalvi T (2015) Detection of some industrially relevant elements in water by electrolyte cathode atmospheric glow discharge optical emission spectrometry. Spectrochim Acta Part B 107:139
Saito G, Akiyama T (2015) Nanomaterial synthesis using plasma generation in liquid. J Nanomater 2015:123696
Chen Q, Li J, Li Y (2015) A review of plasma–liquid interactions for nanomaterial synthesis. J Phys D Appl Phys 48:424005
Horikoshi S, Serpone N (2017) In-liquid plasma: a novel tool in the fabrication of nanomaterials and in the treatment of wastewaters. RSC Adv 7:47196
Rezaei F, Vanraes P, Nikiforov A, Morent R, De Geyter N (2019) Applications of plasma-liquid systems: a review. Materials 12:2751
Sirotkin NA, Khlyustova AV, Titov VA, Krayev AS, Nikitin DI, Dmitrieva OA, Agafonov AV (2020) Synthesis and photocatalytic activity of WO3 nanoparticles prepared by underwater impulse discharge. Plasma Chem Plasma Proc 40:571
Berger S, Tsuchiya H, Ghicov A, Schmuki P (2006) High photocurrent conversion efficiency in self-organized porous WO3. Appl Phys Lett 88:203119
Zhang H, Chen G, Bahnemann DW (2009) Photoelectrocatalytic materials for environmental applications. J Mater Chem 19:5089
Zhang J, Wang XL, Xia XH, Gu CD, Zhao ZJ, Tu JP (2010) Enhanced electrochromic performance of macroporous WO3 films formed by anodic oxidation of DC-sputtered tungsten layers. Electrochim Acta 55:6953
Wang W, Pang YX, Hodgson SNB (2010) Preparation, characterisation and electrochromic property of mesostructured tungsten oxide films via a surfactant templated sol–gel process from tungstic acid. J Sol Gel Sci Technol 54:19
Ippolito SJ, Kandasamy S, Kalantar-zadeh K, Wlodarski W (2005) Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts. Sens Actuators B 108:154
Khlyustova A, Sirotkin N, Titov V, Agafonov A (2021) Effect of low-temperature underwater plasma produced of new properties of Mo–Ti mixed oxide composites for electron transport layer in the dye-sensitized solar cells. J Alloys Comp 858:157664
Reich S, Leitus G, Popovitz-Biro R, Goldbourt A, Vega S (2009) A possible 2D HxWO3 superconductor with a Tc of 120 K. J Supercond Novel Magn 22:343
Sun S, Liao X, Sun Y, Yin G, Yao Y, Huang Z, Pu X (2017) Facile synthesis of a α-MoO3 nanoplate/TiO2 nanotube composite for high electrochemical performance. RSC Adv 7:22983
Liu H, Lv T, Zhu C, Zhu Z (2016) Direct bandgap narrowing of TiO2/MoO3 heterostructure composites for enhanced solar-driven photocatalytic activity. Solar Energy Mater Solar Cells 153:1
Chen CM, Lin ZK, Huang WJ, Yang SH (2016) WO3 nanoparticles or nanorods incorporating Cs2CO3/PCBM buffer bilayer as carriers transporting materials for perovskite solar cells. Nanoscale Res Lett 11:1
Prabhu N, Agilan S, Muthukumarasamy N, Senthil TS (2014) Enhanced photovoltaic performance of WO3 nanoparticles added dye sensitized solar cells. J Mater Sci Mater Electron 25:5288
Wang Z, Hu X, Helmersson U (2000) Peroxo sol–gel preparation: photochromic/electrochromic properties of Mo–Ti oxide gels and thin films. J Mater Chem 10:2396
Jittiarporn P, Sikong L, Kooptarnond K, Taweepreda W, Stoenescu S, Badilescu S, Truong VV (2017) Electrochromic properties of MoO3-WO3 thin films prepared by a sol-gel method, in the presence of a triblock copolymer template. Surf Coat Tech 327:66
Nagyné-Kovács T, Studnicka L, Lukács IE, László K, Pasierb P, Szilágyi IM, Pokol G (2020) Hydrothermal synthesis and gas sensing of monoclinic MoO3 nanosheets. Nanomaterials 10:891
Chen YJ, Xiao G, Wang TS, Zhang F, Ma Y, Gao P, Li QH (2011) α-MoO3/TiO2 core/shell nanorods: controlled-synthesis and low-temperature gas sensing properties. Sens Actuators B Chem 155:270
Khlyustova A, Sirotkin N, Kraev A, Titov V, Agafonov A (2020) Plasma–liquid synthesis of MoOx and WO3 as potential photocatalysts. Dalton Tran 49:6270
Ashkarran AA, Ahadian MM, Ardakani SM (2008) Synthesis and photocatalytic activity of WO3 nanoparticles prepared by the arc discharge method in deionized water. Nanotechnology 19:195709
Khlyustova A, Sirotkin N, Titov V, Agafonov A (2020) Comparison of two types of plasma in contact with water during the formation of molybdenum oxide. Current Appl Phys 20:1396
Kohut A, Ludvigsson L, Meuller BO, Deppert K, Messing ME, Galbács G, Geretovszky Z (2017) From plasma to nanoparticles: optical and particle emission of a spark discharge generator. Nanotechnology 28:475603
Chen A, Su Q, Han H, Enriquez E, Jia Q (2019) Metal oxide nanocomposites: a perspective from strain, defect, and interface. Adv Mater 31:1803241
Khlyustova A, Sirotkin N, Kraev A, Kusova T, Titov V, Agafonov A (2021) Mo doped TiO2 using plasma in contact with liquids: advantages and limitations. J Chem Tech Biotechnol 96:1125
Shaheen I, Ahmad KS, Jaffri SB, Ali D (2021) Biomimetic [MoO3@ZnO] semiconducting nanocomposites: chemo-proportional fabrication, characterization and energy storage potential exploration. Renew Energy 167:568
Bouchard M, Létourneau M, Sarra-Bournet C, Laprise-Pelletier M, Turgeon S, Chevallier P, Fortin MA (2015) Rapid nucleation of iron oxide nanoclusters in aqueous solution by plasma electrochemistry. Langmuir 31:7633
Feng G, Wu B, Khan AQ, Zeng H (2018) In situ glow discharge plasma electrolytic synthesis of reduced TiO2 for enhanced visible light photocatalysis. Mater Res Express 5:055022
Magunov AN (2009) Spectral pyrometry. Instrum Exp Tech 52:451
Hontañón E, Palomares JM, Stein M, Guo X, Engeln R, Nirschl H, Kruis FE (2013) The transition from spark to arc discharge and its implications with respect to nanoparticle production. J Nanoparticle Res 15:1
Miron C, Bratescu MA, Saito N, Takai O (2010) Time-resolved optical emission spectroscopy in water electrical discharges. Plasma Chem Plasma Proc 30:619
Cyber wit diatomic: database and simulation program. www.cyber-wit.com Cited 2011
Griem HR (1974) Spectral line broadening by plasmas. Academic Press, New York
Laux CO, Spence TG, Kruger CH, Zare RN (2003) Optical diagnostics of atmospheric pressure air plasmas. Plasma Sources Sci Tech 12:125
Nikiforov AY, Leys C, Gonzalez MA, Walsh JL (2015) Electron density measurement in atmospheric pressure plasma jets: Stark broadening of hydrogenated and non-hydrogenated lines. Plasma Sources Sci Tech 24:034001
Bruggeman P, Ribežl E, Maslani A, Degroote J, Malesevic A, Rego R, Leys C (2008) Characteristics of atmospheric pressure air discharges with a liquid cathode and a metal anode. Plasma Sources Sci Technol 17:025012
Darwiche S, Nikravech M, Awamat S, Morvan D, Amouroux J (2007) Optical emission spectroscopic investigation of hydrogen plasma used for modification of electrical properties of multi-crystalline silicon. J Phys D Appl Phys 40:1030–1036
Wiese WL, Smith MW, Glennon BM (1966) Atomic transition probabilities. Vol.: hydrogen through Neon. A critical data compilation Washington, DC: National Bureau of Standards
Chauhan V, Gupta T, Koratkar N, Kumar R (2018) Studies of the electronic excitation modifications induced by SHI of Au ions in RF sputtered ZrO2 thin films. Mater Sci Semicond Process 88:262
Xiao L, Zhang S, Huang J (2014) Effective removal of organic dyes by tungstate oxide nanourchins. Powder Tech 258:297
Takahashi Y, Ngaotrakanwiwat P, Tatsuma T (2004) Energy storage TiO2–MoO3 photocatalysts. Electrochim Acta 49:2025
Khan AQ, Yuan S, Niu S, Zheng L, Li W, Zeng H (2017) Synthesis of molybdenum oxide-titanium dioxide nanocomposites with ultrashort laser ablation in water. Opt Exp 25:A539
Xu L, Garrett MP, Hu B (2012) Doping effects on internally coupled seebeck coefficient, electrical, and thermal conductivities in aluminum-doped TiO2. J Phys Chem C 116:13020
Pai DZ (2011) Nanomaterials synthesis at atmospheric pressure using nanosecond discharges. J Phys D Appl Phys 44:174024
Pai DZ, Ostrikov KK, Kumar S, Lacoste DA, Levchenko I, Laux CO (2013) Energy efficiency in nanoscale synthesis using nanosecond plasmas. Sci Rep 3:1
Khlyustova AV, Sirotkin NA, Krayev AS, Titov VA, Agafonov AV (2019) Synthesis of MoO3 by glow discharge in contact with water. Plasma Sci Tech 21:025505
Sirotkin N, Khlyustova A, Titov V, Agafonov A (2020) Plasma-assisted synthesis and deposition of molybdenum oxide nanoparticles on polyethylene terephthalate for photocatalytic degradation of rhodamine B. Plasma Proc Polym 17:2000012
Veklich A, Lebid A, Tmenova T, Boretskij V, Cressault Y, Valensi F, Aftandilyants Y (2017) Plasma assisted generation of micro-and nanoparticles. Plasma Phys Tech 4:28
Acknowledgements
Authors would like to thank the Dr. N. Fomina for conducting XRD analysis and SEM analysis, Dr. N. Kochkina for conducting DLS measurements at the center of joint use of scientific equipment (the Upper Volga Regional Center for Physical-Chemical Research, Russia), Dr. A. Afineevsky for conducting EDS analysis (Interdepartmental Laboratory of Structural Analysis Methods at the Ivanovo State University of Chemistry and Technology) Dr. M. Voronova and Dr. N. Tabachkova for conducting TEM analysis at the center of joint use of scientific equipment at Material Science and Metallurgy, National University of Science and Technology (MISIS, Moscow). This study was performed in the frame of the Government Assignment of the Ministry of Education and Science of Russia (No. 0092-2019-0003) and was in part supported by the Russian Science Foundation under Grant 21-73-00034.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sirotkin, N.A., Khlyustova, A.V., Titov, V.A. et al. The Use of a Novel Three-Electrode Impulse Underwater Discharge for the Synthesis of W-Mo Mixed Oxide Nanocomposites. Plasma Chem Plasma Process 42, 191–209 (2022). https://doi.org/10.1007/s11090-021-10213-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10213-3