Abstract
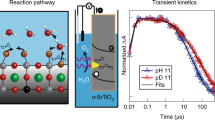
Theoretical descriptors differentiate the catalytic activity of materials for the oxygen evolution reaction by the strength of oxygen binding in the reactive intermediate created upon electron transfer. Recently, time-resolved spectroscopy of a photo-electrochemically driven oxygen evolution reaction followed the vibrational and optical spectra of this intermediate, denoted M-OH*. However, these inherently kinetic experiments have not been connected to the relevant thermodynamic quantities. Here we discover that picosecond optical spectra of the Ti-OH* population on lightly doped SrTiO3 are ordered by the surface hydroxylation. A Langmuir isotherm as a function of pH extracts an effective equilibrium constant relatable to the free energy difference of the first oxygen evolution reaction step. Thus, time-resolved spectroscopy of the catalytic surface reveals both kinetic and energetic information of elementary reaction steps, which provides a critical new connection between theory and experiment by which to tailor the pathway of water oxidation and other surface reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The representative data and all of the analyses from the extended dataset that support the findings of this paper are available in the paper and the Supplementary Information. The extended dataset that supports the findings in this paper is available from the corresponding authors on reasonable request.
Change history
25 November 2021
In the version of this article now appearing online, various notation errors have been corrected to improve readability. No data or conclusions are affected.
References
Hammer, B. & Norskov, J. K. Theoretical surface science and catalysis—calculations and concepts. Adv. Catal. 45, 71–129 (2004).
Markovic, N. M. Electrocatalysis: interfacing electrochemistry. Nat. Mater. 12, 101–102 (2013).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Weckhuysen, B. M. Chemical imaging of spatial heterogeneities in catalytic solids at different length and time scales. Angew. Chem. Int. Ed. 48, 4910–4943 (2009).
Buurmans, I. L. C. & Weckhuysen, B. M. Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nat. Chem. 4, 873–886 (2012).
Rossmeisl, J., Qu, Z. W., Zhu, H., Kroes, G. J. & Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Norskov, J. K. et al. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 37, 2163–2171 (2008).
Zhang, M. & Frei, H. Towards a molecular level understanding of the multi-electron catalysis of water oxidation on metal oxide surfaces. Catal. Lett. 145, 420–435 (2014).
Kern, J. et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563, 421–425 (2018).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Busch, M. et al. Beyond the top of the volcano? – A unified approach to electrocatalytic oxygen reduction and oxygen evolution. Nano Energy 29, 126–135 (2016).
Chen, X. et al. The formation time of Ti–O• and Ti–O•–Ti radicals at the n-SrTiO3/aqueous interface during photocatalytic water oxidation. J. Am. Chem. Soc. 139, 1830–1841 (2017).
Herlihy, D. M. et al. Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration. Nat. Chem. 8, 549–555 (2016).
Zhang, M., de Respinis, M. & Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem. 6, 362–367 (2014).
Zandi, O. & Hamann, T. W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 8, 778–783 (2016).
Cheng, J., VandeVondele, J. & Sprik, M. Identifying trapped electronic holes at the aqueous TiO2 interface. J. Phys. Chem. C 118, 5437–5444 (2014).
Chen, J., Li, Y. F., Sit, P. & Selloni, A. Chemical dynamics of the first proton-coupled electron transfer of water oxidation on TiO2 anatase. J. Am. Chem. Soc. 135, 18774–18777 (2013).
Kraack, J. P. & Hamm, P. Surface-sensitive and surface-specific ultrafast two-dimensional vibrational spectroscopy. Chem. Rev. 117, 10623–10664 (2017).
Kraack, J. P., Kaech, A. & Hamm, P. Surface enhancement in ultrafast 2D ATR IR spectroscopy at the metal-liquid interface. J. Phys. Chem. C 120, 3350–3359 (2016).
Klahr, B., Gimenez, S., Fabregat-Santiago, F., Bisquert, J. & Hamann, T. W. Photoelectrochemical and impedance spectroscopic investigation of water oxidation with “Co-Pi”-coated hematite electrodes. J. Am. Chem. Soc. 134, 16693–16700 (2012).
Chen, X., Aschaffenburg, D. J. & Cuk, T. Selecting between two transition states by which water oxidation intermediates decay on an oxide surface. Nat. Catal. 2, 820–827 (2019).
Libuda, J., Meusel, I., Hartmann, J. & Freund, H. J. A molecular beam/surface spectroscopy apparatus for the study of reactions on complex model catalysts. Rev. Sci. Instrum. 71, 4395–4408 (2000).
Parsons, R. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Faraday Soc. 54, 1053–1063 (1958).
Zhang, Y. et al. A review on thermalization mechanisms and prospect absorber materials for the hot carrier solar cells. Sol. Energy Mater. Sol. Cells 225, 111073 (2021).
Bhattacharya, C. et al. Sustainable nanoplasmon-enhanced photoredox reactions: synthesis, characterization, and applications. Adv. Energy Mater. 10, 2002402 (2020).
Xu, Y. & Schoonen, M. A. A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 85, 543–556 (2000).
Janotti, A., Varley, J. B., Choi, M. & Van de Walle, C. G. Vacancies and small polarons in SrTiO3. Phys. Rev. B 90, 085202 (2014).
Chen, H. & Umezawa, N. Hole localization, migration, and the formation of peroxide anion in perovskite SrTiO3. Phys. Rev. B 90, 035202 (2014).
Rubano, A., Paparo, D., Granozio, F. M., Uccio, U. & Marrucci, L. Blue luminescence of SrTiO3 under intense optical excitation. J. Appl. Phys. 106, 103515 (2009).
Mochizuki, S., Fujishiro, F. & Minami, S. Photoluminescence and reversible photo-induced spectral change of SrTiO3. J. Phys. Condens. Matter 17, 923–948 (2005).
Schirmer, O. F. O− bound small polarons in oxide materials. J. Phys. Condens. Matter 18, R667–R704 (2006).
Deskins, N. A. & Dupuis, M. Intrinsic hole migration rates in TiO2 from density functional theory. J. Phys. Chem. C 113, 346–358 (2009).
Formal, F. L. et al. Rate law analysis of water oxidation on a hematite surface. J. Am. Chem. Soc. 137, 6629–6637 (2015).
Kafizas, A. et al. Water oxidation kinetics of accumulated holes on the surface of a TiO2 photoanode: a rate law analysis. ACS Catal. 7, 4896–4903 (2017).
Waegele, M. M., Chen, X., Herlihy, D. M. & Cuk, T. How surface potential determines the kinetics of the first hole transfer of photocatalytic water oxidation. J. Am. Chem. Soc. 136, 10632–10639 (2014).
Mandal, A., Ramasesha, K., Marco, L. D. & Tokmakoff, A. Collective vibrations of water-solvated hydroxide ions investigated with broadband 2DIR spectroscopy. J. Chem. Phys. 140, 204508 (2014).
Baiz, C. R., Peng, C. S., Reppert, M. E., Jones, K. C. & Tokmakoff, A. Coherent two-dimensional infrared spectroscopy: quantitative analysis of protein secondary structure in solution. Analyst 137, 1793–1799 (2012).
Stewart, G. W. On the early history of the singular value decomposition. SIAM Rev. 35, 551–566 (1993).
Guhl, H., Miller, W. & Reuter, K. Water adsorption and dissociation on SrTiO3 (001) revisited: a density functional theory study. Phys. Rev. B 81, 155455 (2010).
Hinojosa, B. B., Van Cleve, T. & Asthagiri, A. A first-principles study of H2O adsorption and dissociation on the SrTiO3(100) surface. Mol. Simul. 36, 604–617 (2010).
Holmstrom, A., Spijker, P. & Foster, A. S. The interface of SrTiO2 and H2O from density functional theory molecular dynamics. Proc. R. Soc. A 472, 20160293 (2016).
Roberts, S. T., Ramasesha, K., Petersen, P. B., Mandal, A. & Tokmakoff, A. Proton transfer in concentrated aqueous hydroxide visualized using ultrafast infrared spectroscopy. J. Phys. Chem. A 115, 3957–3972 (2011).
Marx, D., Chandra, A. & Tuckerman, M. E. Aqueous basic solutions: hydroxide solvation, structural diffusion, and comparison to the hydrated proton. Chem. Rev. 110, 2174–2216 (2010).
Perakis, F., Widmer, S. & Hamm, P. Two-dimensional infrared spectroscopy of isotope-diluted ice Ih. J. Chem. Phys. 134, 204505 (2011).
De Marco, L. et al. Differences in the vibrational dynamics of H2O and D2O: observation of symmetric and antisymmetric stretching vibrations in heavy water. J. Phys. Chem. Lett. 7, 1769–1774 (2016).
Selcuk, S. & Selloni, A. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces. Nat. Mater. 15, 1107–1112 (2016).
Swenson, H. & Stadie, N. P. Langmuir’s theory of adsorption: a centennial review. Langmuir 35, 5409–5426 (2019).
Cheng, J. & Sprik, M. Acidity of the aqueous rutile TiO2(110) surface from density functional theory based molecular dynamics. J. Chem. Theory Comput. 6, 880–889 (2010).
Sanchez, E. K. et al. Assessment of polishing-related surface damage in silicon carbide. J. Electrochem. Soc. 149, G131–G136 (2002).
Arnay, I., Rubio-Zuazo, J. & Castro, G. R. Impact of cleaning methods on the structural properties and morphology of SrTiO3 surface. Appl. Surf. Sci. 427, 561–565 (2018).
Feng, Y., Vinogradov, I. & Ge, N.-H. General noise suppression scheme with reference detection in heterodyne nonlinear spectroscopy. Opt. Express 25, 26262–26279 (2017).
Feng, Y., Vinogradov, I. & Ge, N.-H. Optimized noise reduction scheme for heterodyne spectroscopy using array detectors. Opt. Express 27, 20323–20346 (2019).
Acknowledgements
The experimental work was supported by the Director, Office of Science, Office of Basic Energy Sciences and by the Division of Chemical Sciences, Geosciences and Biosciences of the US Department of Energy at the Renewable and Sustainable Energy Institute (RASEI; Boulder, CO) under contract no. DE-SC0018939 awarded to T.C. This included full support for one postdoctoral fellow and one graduate student, and partial support for another graduate student. We thank C. D. Pemmaraju and H. Frei for helpful discussions.
Author information
Authors and Affiliations
Contributions
T.C. conceived of the project, managed the data collection process and wrote the manuscript with the input of all authors. I.V., S.S. and H.L. constructed the transient set-up and collected the transient data. M.P. helped collect the transient data on the highly doped samples. I.V. and A.M. processed the results and analysed the data using principal component analyses. T.C., with input from J.R., developed the theoretical model. J.R. placed the results in the context of previous theoretical models.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks Bert Weckhuysen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18 and Sections I–X.
Rights and permissions
About this article
Cite this article
Vinogradov, I., Singh, S., Lyle, H. et al. Free energy difference to create the M-OH* intermediate of the oxygen evolution reaction by time-resolved optical spectroscopy. Nat. Mater. 21, 88–94 (2022). https://doi.org/10.1038/s41563-021-01118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01118-9
This article is cited by
-
Understanding the sulphur-oxygen exchange process of metal sulphides prior to oxygen evolution reaction
Nature Communications (2023)
-
Potential-dependent transition of reaction mechanisms for oxygen evolution on layered double hydroxides
Nature Communications (2023)
-
Recent advances of hydrogen production through particulate semiconductor photocatalytic overall water splitting
Frontiers in Energy (2022)