Abstract
Cancer development is a dynamic evolutionary process characterized by marked intratumoural heterogeneity at the genetic, epigenetic and phenotypic levels. Barrett oesophagus, the pre-malignant condition to oesophageal adenocarcinoma (EAC), is an exemplary system to longitudinally study the evolution of malignancy. Evidence has emerged of Barrett oesophagus lesions pre-programmed for progression to EAC many years before clinical detection, indicating a considerable window for therapeutic intervention. In this Review, we explore the mechanisms underlying clonal expansion and contraction that establish the Barrett oesophagus clonal mosaicism over time and space and discuss intrinsic genotypic and extrinsic environmental drivers that direct the evolutionary trajectory of Barrett oesophagus towards a malignant phenotype. We propose that understanding and exploiting the evolutionary dynamics of Barrett oesophagus will identify novel therapeutic targets, improve prognostic tools and offer the opportunity for personalized surveillance programmes geared to prevent progression to EAC.
Key points
-
Longitudinal surveillance of Barrett oesophagus offers an exemplary opportunity to study lesion evolution over time and space and during the progression to oesophageal adenocarcinoma.
-
Distinct evolutionary patterns exist between non-progressing and progressing Barrett oesophagus with evidence for protracted evolutionary dynamics occurring over many years.
-
Chronic inflammation due to reflux accelerates evolution through mutagenesis and provides a selective pressure for mutant clones that are viable in the harsh environment and can rapidly repopulate the ulcerated mucosa.
-
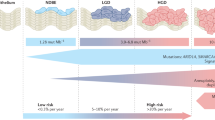
The measurement of evolutionary dynamics in Barrett oesophagus lesions is a potentially powerful prognostic tool and identifies a wide window for therapeutic intervention and prevention of cancer in patients with Barrett oesophagus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cook, M. B., Chow, W. H. & Devesa, S. S. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br. J. Cancer 101, 855–859 (2009).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Arnold, M. et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 20, 1493–1505 (2019).
Morgan, E. et al. International trends in oesophageal cancer survival by histological subtype between 1995 and 2014. Gut 70, 234–242 (2021).
Arnold, M., Ferlay, J., van Berge Henegouwen, M. I. & Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69, 1564–1571 (2020).
Arnold, M., Laversanne, M., Brown, L. M., Devesa, S. S. & Bray, F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am. J. Gastroenterol. 112, 1247–1255 (2017). Predictions fitting and extrapolating age–period–cohort models expect the number of new EAC cases to increase rapidly between 2005 and 2030, thereby becoming the predominant type of oesophageal cancer in a growing number of high-income countries.
Smyth, E. C. et al. Oesophageal cancer. Nat. Rev. Dis. Prim. 3, 17048 (2017).
Naini, B. V., Souza, R. F. & Odze, R. D. Barrett’s esophagus: a comprehensive and contemporary review for pathologists. Am. J. Surg. Pathol. 40, e45–e66 (2016).
Spechler, S. J., Fitzgerald, R. C., Prasad, G. A. & Wang, K. K. History, molecular mechanisms, and endoscopic treatment of Barrett’s esophagus. Gastroenterology 138, 854–869 (2010).
Ronkainen, J. et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 129, 1825–1831 (2005).
Zagari, R. M. et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut 57, 1354–1359 (2008).
Runge, T. M., Abrams, J. A. & Shaheen, N. J. Epidemiology of Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol. Clin. North. Am. 44, 203–231 (2015).
Fitzgerald, R. C. et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 396, 333–344 (2020). A multicentre randomized controlled trial showed superior performance of Barrett oesophagus detection in the primary care setting using a novel non-endoscopic Cytosponge-TFF3 device compared with standard care in the primary care setting of patients with gastro-oesophageal reflux.
Shaheen, N. J. & Richter, J. E. Barrett’s oesophagus. Lancet 373, 850–861 (2009).
Hamade, N. et al. Significant decline in the prevalence of Barrett’s esophagus among patients with gastroesophageal reflux disease. Dis. Esophagus 34, doaa131 (2021).
O’Donovan, M. & Fitzgerald, R. C. Screening for Barrett’s esophagus: are new high-volume methods feasible? Dig. Dis. Sci. 63, 2105–2114 (2018).
Harrison, R. et al. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am. J. Gastroenterol. 102, 1154–1161 (2007).
Weusten, B. et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 49, 191–198 (2017).
Shaheen, N. J., Falk, G. W., Iyer, P. G. & Gerson, L. B., American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 111, 30–50 (2016).
di Pietro, M., Alzoubaidi, D. & Fitzgerald, R. C. Barrett’s esophagus and cancer risk: how research advances can impact clinical practice. Gut Liver 8, 356–370 (2014).
Levine, D. S., Blount, P. L., Rudolph, R. E. & Reid, B. J. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am. J. Gastroenterol. 95, 1152–1157 (2000).
Sharma, P. et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 131, 1392–1399 (2006).
Pohl, H. et al. Length of Barrett’s oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut 65, 196–201 (2016).
Antony, A. et al. Adherence to quality indicators in endoscopic surveillance of Barrett’s esophagus and correlation to dysplasia detection rates. Clin. Res. Hepatol. Gastroenterol. 42, 591–596 (2018).
Verbeek, R. E. et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am. J. Gastroenterol. 109, 1215–1222 (2014).
Holmberg, D., Ness-Jensen, E., Mattsson, F. & Lagergren, J. Adherence to clinical guidelines for Barrett’s esophagus. Scand. J. Gastroenterol. 54, 945–952 (2019).
Peters, F. P. et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest. Endosc. 67, 604–609 (2008).
Schlemper, R. J. et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47, 251–255 (2000).
Fitzgerald, R. C. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 63, 7–42 (2014).
Vennalaganti, P. et al. Discordance among pathologists in the United States and Europe in diagnosis of low-grade dysplasia for patients with Barrett’s esophagus. Gastroenterology 152, 564–570.e4 (2017).
Salomao, M. A., Lam-Himlin, D. & Pai, R. K. Substantial interobserver agreement in the diagnosis of dysplasia in Barrett esophagus upon review of a patient’s entire set of biopsies. Am. J. Surg. Pathol. 42, 376–381 (2018).
Montgomery, E. et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum. Pathol. 32, 368–378 (2001).
Spechler, S. J., Sharma, P., Souza, R. F., Inadomi, J. M. & Shaheen, N. J. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 140, 1084–1091 (2011).
Hvid-Jensen, F., Pedersen, L., Drewes, A. M., Sørensen, H. T. & Funch-Jensen, P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 365, 1375–1383 (2011).
Hvid-Jensen, F. & Drewes, A. M. Should aspirin and PPIs be recommended for patients with Barrett’s oesophagus? Lancet 392, 362–364 (2018).
Zeki, S. & Fitzgerald, R. C. Targeting care in Barrett’s oesophagus. Clin. Med. 14 (Suppl. 6), s78–s83 (2014).
Wenker, T. N., Tan, M. C., Liu, Y., El-Serag, H. B. & Thrift, A. P. Prior diagnosis of Barrett’s esophagus is infrequent, but associated with improved esophageal adenocarcinoma survival. Dig. Dis. Sci. 63, 3112–3119 (2018).
Visrodia, K. et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: a systematic review and meta-analysis. Gastroenterology 150, 599–607.e7 (2016).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012). This Review explains fundamental principles of clonal evolution in cancer such as genetic diversification, genetic drivers, the clonal architecture, clonal dynamics and the influence of the cancer ecosystem.
Nik-Zainal, S. et al. The life history of 21 breast cancers. Cell 149, 994–1007 (2012).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Sottoriva, A. et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl Acad. Sci. USA 110, 4009–4014 (2013).
de Bruin, E. C. et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 346, 251–256 (2014).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Landau, D. A. et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152, 714–726 (2013).
Merlo, L. M. F., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Souza, R. F. Reflux esophagitis and its role in the pathogenesis of Barrett’s metaplasia. J. Gastroenterol. 52, 767–776 (2017).
McQuaid, K. R., Laine, L., Fennerty, M. B., Souza, R. & Spechler, S. J. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment. Pharmacol. Ther. 34, 146–165 (2011).
Que, J., Garman, K. S., Souza, R. F. & Spechler, S. J. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 157, 349–364.e1 (2019).
Jiang, M. et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550, 529–533 (2017).
Leedham, S. J. et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut 57, 1041–1048 (2008).
von Furstenberg, R. J. et al. Porcine esophageal submucosal gland culture model shows capacity for proliferation and differentiation. Cell Mol. Gastroenterol. Hepatol. 4, 385–404 (2017).
Wang, X. et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 145, 1023–1035 (2011).
Nowicki-Osuch, K. et al. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science 373, 760–767 (2021).
Peters, Y. et al. Barrett oesophagus. Nat. Rev. Dis. Prim. 5, 35 (2019).
Galipeau, P. C., Prevo, L. J., Sanchez, C. A., Longton, G. M. & Reid, B. J. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J. Natl Cancer Inst. 91, 2087–2095 (1999).
Wong, D. J. et al. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 61, 8284–8289 (2001).
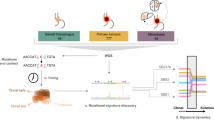
Martinez, P. et al. Dynamic clonal equilibrium and predetermined cancer risk in Barrett’s oesophagus. Nat. Commun. 7, 12158 (2016). A longitudinal single-cell fluorescent in situ hybridization analysis of non-dysplastic Barrett oesophagus biopsy samples, including 300 non-progressing and 20 progressing Barrett oesophagus lesions, showing that clones exist in a dynamic equilibrium with relatively stable levels of genetic diversity over time, whereby clonal expansions are rare events.
Li, X. et al. Assessment of esophageal adenocarcinoma risk using somatic chromosome alterations in longitudinal samples in Barrett’s esophagus. Cancer Prev. Res. 8, 845–856 (2015). A multi-region SNP array analysis of CNAs in 248 patients with Barrett oesophagus over space and time showing that non-progressing Barrett oesophagus typically harbours a low burden of large-scale CNAs that remain low over time, whereas progressing Barrett oesophagus develops high levels of CNAs.
Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat. Genet. 38, 468–473 (2006). A fluorescent in situ hybridization study including 268 patients with non-progressing Barrett oesophagus and 37 patients with progressing Barrett oesophagus identifies genetic clonal diversity as a predictor of progression to EAC in patients with Barrett oesophagus.
Ross-Innes, C. S. et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat. Genet. 47, 1038–1046 (2015). Whole-genome sequencing on 23 paired Barrett oesophagus and EAC samples and one in-depth Barrett oesophagus case sampled over time and space reveals the presence of mutations and copy number changes in non-dysplastic Barrett prior to the phenotypic evolution of the clonally related HGD descendent, questioning the adequacy of dysplasia as a biomarker for cancer risk.
Slaughter, D. P., Southwick, H. W. & Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968 (1953).
Braakhuis, B. J. M., Tabor, M. P., Kummer, J. A., Leemans, C. R. & Brakenhoff, R. H. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 63, 1727–1730 (2003).
Galandiuk, S. et al. Field cancerization in the intestinal epithelium of patients with Crohn’s ileocolitis. Gastroenterology 142, 855–864.e8 (2012).
Eads, C. A. et al. Fields of aberrant CpG island hypermethylation in Barrett’s esophagus and associated adenocarcinoma. Cancer Res. 60, 5021–5026 (2000).
Gu, J. et al. Genome-wide catalogue of chromosomal aberrations in Barrett’s esophagus and esophageal adenocarcinoma: a high-density single nucleotide polymorphism array analysis. Cancer Prev. Res. 3, 1176–1186 (2010).
Reid, B. J. et al. Barrett’s esophagus: ordering the events that lead to cancer. Eur. J. Cancer Prev. 5 (Suppl. 2), 57–65 (1996).
Li, X. et al. Temporal and spatial evolution of somatic chromosomal alterations: a case-cohort study of Barrett’s esophagus. Cancer Prev. Res. 7, 114–127 (2014). A large longitudinal study that examines multi-region CNA profiles of 79 patients with Barrett oesophagus that progressed to EAC and 169 patients with Barrett oesophagus that did not progress and showed that progressing Barrett oesophagus has more CNAs than non-progressing Barrett oesophagus and that progressors display a sudden sharp increase in genetic alterations 2–4 years before cancer detection.
Hardie, L. J. et al. p16 expression in Barrett’s esophagus and esophageal adenocarcinoma: association with genetic and epigenetic alterations. Cancer Lett. 217, 221–230 (2005).
Bian, Y.-S., Osterheld, M.-C., Fontolliet, C., Bosman, F. T. & Benhattar, J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett’s esophagus. Gastroenterology 122, 1113–1121 (2002).
Stachler, M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 47, 1047–1055 (2015). A paired whole-exome study of Barrett oesophagus and EAC suggesting that many EACs emerge through TP53 mutation with subsequent genome doubling, followed by the acquisition of oncogenic amplifications.
Weaver, J. M. J. et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat. Genet. 46, 837–843 (2014). A whole-genome sequencing study including EAC, high-grade dysplasia and non-dysplastic Barrett oesophagus shows that only mutations in TP53 and SMAD4 occur in a stage-specific manner, whereas the majority of recurrently mutated genes in EAC are also mutated in non-dysplastic Barrett oesophagus and might therefore occur early in Barrett development.
Dulak, A. M. et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 45, 478–486 (2013).
Galipeau, P. C. et al. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett’s esophagus. Proc. Natl Acad. Sci. USA 93, 7081–7084 (1996).
Reid, B. J. et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. Am. J. Gastroenterol. 96, 2839–2848 (2001).
Maley, C. C. et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 64, 7629–7633 (2004).
Killcoyne, S. et al. Genomic copy number predicts esophageal cancer years before transformation. Nat. Med. 26, 1726–1732 (2020). Using shallow whole-genome sequencing of 777 Barrett oesophagus biopsy samples from a retrospective cohort of 88 patients undergoing Barrett oesophagus surveillance, a risk stratification model based on CNA profile complexity was able to identify 50% of patients at high risk ≥8 years before clinical progression and showed improving detection rates to 78% and 85% at 2 years and 1 year prior, respectively.
Quante, M., Graham, T. A. & Jansen, M. Insights into the pathophysiology of esophageal adenocarcinoma. Gastroenterology 154, 406–420 (2018).
McDonald, S. A. C. et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology 134, 500–510 (2008).
Theodorou, D. et al. Intraluminal pH and goblet cell density in Barrett’s esophagus. J. Gastrointest. Surg. 16, 469–474 (2012).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012). In a transgenic mouse model of Barrett oesophagus, oesophageal overexpression of IL-1β led to oesophagitis, Barrett-like metaplasia and EAC, which could be accelerated by exposure to bile acids and nitrosamines.
O’Riordan, J. M. et al. Factors influencing the development of Barrett’s epithelium in the esophageal remnant postesophagectomy. Am. J. Gastroenterol. 99, 205–211 (2004).
Lavery, D. L. et al. Evolution of oesophageal adenocarcinoma from metaplastic columnar epithelium without goblet cells in Barrett’s oesophagus. Gut 65, 907–913 (2016).
Khan, S. et al. Crypt dysplasia in Barrett’s oesophagus shows clonal identity between crypt and surface cells. J. Pathol. 231, 98–104 (2013).
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Graham, T. A. & Sottoriva, A. Measuring cancer evolution from the genome. J. Pathol. 241, 183–191 (2017).
Eldredge, N. & Gould, S. J. On punctuated equilibria. Science 276, 338–341 (1997).
Newell, F. et al. Complex structural rearrangements are present in high-grade dysplastic Barrett’s oesophagus samples. BMC Med. Genomics 12, 31 (2019).
Goldschmidt, R. The Material Basis of Evolution (Yale Univ. Press, 1940).
Carter, S. L. et al. Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol. 30, 413–421 (2012).
Nones, K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 5, 5224 (2014).
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Notta, F. et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 538, 378–382 (2016).
Malhotra, A. et al. Breakpoint profiling of 64 cancer genomes reveals numerous complex rearrangements spawned by homology-independent mechanisms. Genome Res. 23, 762–776 (2013).
Rausch, T. et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 148, 59–71 (2012).
Przybytkowski, E. et al. Chromosome-breakage genomic instability and chromothripsis in breast cancer. BMC Genomics 15, 579 (2014).
Finley, J. C. et al. Chromosomal instability in Barrett’s esophagus is related to telomere shortening. Cancer Epidemiol. Biomark. Prev. 15, 1451–1457 (2006).
Cook, M. B. et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J. Natl Cancer Inst. 102, 1344–1353 (2010).
Coleman, H. G. et al. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett’s esophagus. Gastroenterology 142, 233–240 (2012).
Olliver, J. R. et al. Risk factors, DNA damage, and disease progression in Barrett’s esophagus. Cancer Epidemiol. Biomark. Prev. 14, 620–625 (2005).
Kaz, A. M. et al. Global DNA methylation patterns in Barrett’s esophagus, dysplastic Barrett’s, and esophageal adenocarcinoma are associated with BMI, gender, and tobacco use. Clin. Epigenetics 8, 111 (2016).
Kadakia, S. C., De La Baume, H. R. & Shaffer, R. T. Effects of transdermal nicotine on lower esophageal sphincter and esophageal motility. Dig. Dis. Sci. 41, 2130–2134 (1996).
Nieto, T. et al. A systematic review of epigenetic biomarkers in progression from non-dysplastic Barrett’s oesophagus to oesophageal adenocarcinoma. BMJ Open 8, e020427 (2018).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Taniguchi, K. & Karin, M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Thrift, A. P., Garcia, J. M. & El-Serag, H. B. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 12, 1267–1271 (2014).
Lagergren, J., Bergström, R., Lindgren, A. & Nyrén, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 340, 825–831 (1999).
Schmidt, M. et al. Epidemiologic risk factors in a comparison of a Barrett Esophagus Registry (BarrettNET) and a case-control population in Germany. Cancer Prev. Res. 13, 377–384 (2020).
Parsonnet, J. Molecular mechanisms for inflammation-promoted pathogenesis of cancer — The Sixteenth International Symposium of the Sapporo Cancer Seminar. Cancer Res. 57, 3620–3624 (1997).
Feagins, L. A. et al. Mechanisms of oxidant production in esophageal squamous cell and Barrett’s cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G411–G417 (2008).
Secrier, M. et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat. Genet. 48, 1131–1141 (2016).
Fitzgerald, R. C. et al. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50, 451–459 (2002).
Fitzgerald, R. C. et al. Inflammatory gradient in Barrett’s oesophagus: implications for disease complications. Gut 51, 316–322 (2002). This article shows that specific cytokine responses with pro-inflammatory IL-8 and IL-1β expression might contribute to the local inflammatory microenvironment in Barrett oesophagus.
Kavanagh, M. E. et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 370, 117–124 (2016).
Wolfsen, H. C., Hemminger, L. L. & DeVault, K. R. Recurrent Barrett’s esophagus and adenocarcinoma after esophagectomy. BMC Gastroenterol. 4, 18 (2004).
Oberg, S., Johansson, J., Wenner, J. & Walther, B. Metaplastic columnar mucosa in the cervical esophagus after esophagectomy. Ann. Surg. 235, 338–345 (2002).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Tsilimigras, M. C. B., Fodor, A. & Jobin, C. Carcinogenesis and therapeutics: the microbiota perspective. Nat. Microbiol. 2, 17008 (2017).
Yang, L. et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137, 588–597 (2009).
Elliott, D. R. F., Walker, A. W., O’Donovan, M., Parkhill, J. & Fitzgerald, R. C. A non-endoscopic device to sample the oesophageal microbiota: a case-control study. Lancet Gastroenterol. Hepatol. 2, 32–42 (2017).
Yamamura, K. et al. Human microbiome fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 22, 5574–5581 (2016).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
McCoy, A. N. et al. Fusobacterium is associated with colorectal adenomas. PLoS ONE 8, e53653 (2013).
Castellarin, M. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306 (2012).
Jankowski, J. A. Z. et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet 392, 400–408 (2018). A randomized controlled trial comparing permutations of low-dose and high-dose PPIs with or without aspirin as a chemopreventive strategy in patients with Barrett oesophagus shows a statistically significant benefit of high-dose over low-dose PPIs, which could be enhanced with use of aspirin with high-dose PPIs.
Jovani, M. et al. Aspirin use is associated with lower risk of Barrett’s esophagus in women. Clin. Transl. Gastroenterol. 8, e131 (2017).
Galipeau, P. C. et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 4, e67 (2007).
Rothwell, P. M. et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41 (2011).
Baron, J. A. et al. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 348, 891–899 (2003).
Flossmann, E. & Rothwell, P. M. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369, 1603–1613 (2007).
Algra, A. M. & Rothwell, P. M. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 13, 518–527 (2012).
Katona, B. W. & Weiss, J. M. Chemoprevention of colorectal cancer. Gastroenterology 158, 368–388 (2020).
Picardo, S. L., Maher, S. G., O’Sullivan, J. N. & Reynolds, J. V. Barrett’s to oesophageal cancer sequence: a model of inflammatory-driven upper gastrointestinal cancer. Dig. Surg. 29, 251–260 (2012).
Galipeau, P. C. et al. NSAID use and somatic exomic mutations in Barrett’s esophagus. Genome Med. 10, 17 (2018).
Kostadinov, R. L. et al. NSAIDs modulate clonal evolution in Barrett’s esophagus. PLoS Genet. 9, e1003553 (2013).
Bhat, S. et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J. Natl Cancer Inst. 103, 1049–1057 (2011).
van der Wel, M. J. et al. Improved diagnostic stratification of digitised Barrett’s oesophagus biopsies by p53 immunohistochemical staining. Histopathology 72, 1015–1023 (2018).
Kaye, P. V. et al. Dysplasia in Barrett’s oesophagus: p53 immunostaining is more reproducible than haematoxylin and eosin diagnosis and improves overall reliability, while grading is poorly reproducible. Histopathology 69, 431–440 (2016). In this study, p53 IHC is reported as having increased reliability and inter-observer reliability in the diagnosis of dysplasia but showing poor results in discrimination between LGD and HGD.
Spechler, S. J. et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 140, e18–e52 (2011).
Varghese, S., Lao-Sirieix, P. & Fitzgerald, R. C. Identification and clinical implementation of biomarkers for Barrett’s esophagus. Gastroenterology 142, 435–441.e2 (2012).
Stachler, M. D. et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology 155, 156–167 (2018).
Bird-Lieberman, E. L. et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology 143, 927–935.e3 (2012).
Kastelein, F. et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut 62, 1676–1683 (2013). This paper shows that aberrant p53 IHC staining is associated with an increased risk of neoplastic progression in patients with Barrett oesophagus and increased positive predictive value for neoplastic progression from 15% with histological diagnosis of LGD alone to 33% with LGD and concurrent aberrant p53 expression.
Hadjinicolaou, A. V. et al. Aneuploidy in targeted endoscopic biopsies outperforms other tissue biomarkers in the prediction of histologic progression of Barrett’s oesophagus: a multi-centre prospective cohort study. EBioMedicine 56, 102765 (2020).
Sharma, P., Shaheen, N. J., Katzka, D. & Bergman, J. AGA Clinical Practice Update on Endoscopic Treatment of Barrett’s Esophagus with Dysplasia and/or Early Cancer: Expert Review. Gastroenterology 158, 760–769 (2020).
van Olphen, S. et al. SOX2 as a novel marker to predict neoplastic progression in Barrett’s esophagus. Am. J. Gastroenterol. 110, 1420–1428 (2015).
Hong, M. K. et al. Expansion of the Ki-67 proliferative compartment correlates with degree of dysplasia in Barrett’s esophagus. Cancer 75, 423–429 (1995).
Sikkema, M. et al. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett’s esophagus: a case-control study. Am. J. Gastroenterol. 104, 2673–2680 (2009).
Yousaf, H. et al. Surface Ki-67 expression improves reproducibility of dysplasia diagnosis in Barrett’s esophagus. Am. J. Clin. Pathol. 153, 695–704 (2020).
Altaf, K., Xiong, J. J., la Iglesia, D., Hickey, L. & Kaul, A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s oesophagus. Br. J. Surg. 104, 493–502 (2017).
Findlay, J. M., Middleton, M. R. & Tomlinson, I. Genetic biomarkers of Barrett’s esophagus susceptibility and progression to dysplasia and cancer: a systematic review and meta-analysis. Dig. Dis. Sci. 61, 25–38 (2016).
Choi, W. T. et al. Diagnosis and risk stratification of Barrett’s dysplasia by flow cytometric DNA analysis of paraffin-embedded tissue. Gut 67, 1229–1238 (2017).
Timmer, M. R. et al. Derivation of genetic biomarkers for cancer risk stratification in Barrett’s oesophagus: a prospective cohort study. Gut 65, 1602–1610 (2016).
Hoefnagel, S. J. M. et al. A genomic biomarker-based model for cancer risk stratification of non-dysplastic Barrett’s esophagus patients after extended follow up; results from Dutch surveillance cohorts. PLoS ONE 15, e0231419 (2020).
Rabinovitch, P. S., Longton, G., Blount, P. L., Levine, D. S. & Reid, B. J. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am. J. Gastroenterol. 96, 3071–3083 (2001).
Reid, B. J., Levine, D. S., Longton, G., Blount, P. L. & Rabinovitch, P. S. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am. J. Gastroenterol. 95, 1669–1676 (2000).
Curtius, K., Wright, N. A. & Graham, T. A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 18, 19–32 (2018).
Tschanz, E. R. Do 40% of patients resected for Barrett esophagus with high-grade dysplasia have unsuspected adenocarcinoma? Arch. Pathol. Lab. Med. 129, 177–180 (2005).
Corley, D. A. et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology 145, 312–319.e1 (2013).
Sikkema, M., de Jonge, P. J., Steyerberg, E. W. & Kuipers, E. J. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 8, 235–244 (2010). quiz e232.
Martinez, P. et al. Evolution of Barrett’s esophagus through space and time at single-crypt and whole-biopsy levels. Nat. Commun. 9, 794 (2018).
Ross-Innes, C. S. et al. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol. Hepatol. 2, 23–31 (2017).
Moinova, H. R. et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 10, eaao5848 (2018).
Jin, Z. et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 69, 4112–4115 (2009).
Alvi, M. A. et al. DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett’s esophagus. Clin. Cancer Res. 19, 878–888 (2013).
Kaz, A. M. et al. DNA methylation profiling in Barrett’s esophagus and esophageal adenocarcinoma reveals unique methylation signatures and molecular subclasses. Epigenetics 6, 1403–1412 (2011).
Iyer, P. G. et al. Highly discriminant methylated DNA markers for the non-endoscopic detection of Barrett’s esophagus. Am. J. Gastroenterol. 113, 1156–1166 (2018).
Wang, Z. et al. Methylation biomarker panel performance in esophacap cytology samples for diagnosing Barrett’s esophagus: a prospective validation study. Clin. Cancer Res. 25, 2127–2135 (2019).
Maley, C. C. et al. Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett’s esophagus. Cancer Res. 64, 3414–3427 (2004).
Acknowledgements
The authors acknowledge funding from: German Cancer Aid Society (Deutsche Krebshilfe) (M.S.), Cancer Research UK (R.J.H.), Cancer Research UK (A19771) (A.B. and T.A.G.), the US NIH via the Cancer Systems Biology Consortium U54 scheme (CA217376) (T.A.G.), German Research Foundation (DFG 3772/1) (M.Q.), Cancer Research UK PFA award (A21446) (S.A.C.M.) and a Cancer Research UK Grand Challenge award (STORMing Cancer, A29071) (S.A.C.M.).
Author information
Authors and Affiliations
Contributions
M.S. and R.J.H. wrote the first draft of the review. A.B., S.A.C.M., M.Q. and T.A.G. edited the review. All authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Rebecca Fitzgerald and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Dysplasia
-
Neoplastic epithelium that remains confined within the basement membrane of the epithelium.
- Fitness
-
The average contribution of a genotype to the next generation. The fitness of a genotype is manifested through its phenotype, which is also affected by the environment. Fitness generally promotes both survival and reproduction.
- Clonal expansion
-
Spatial expansion of a clone through increased proliferation due to an advantageous phenotype.
- Genetic diversity
-
The multitude of genetic variation on which selection can act.
- Intratumoural heterogeneity
-
Diversity within individual tumours at the genetic, epigenetic and transcriptomic level.
- Clone
-
A group of cells that share a common genotype as they descend from a common ancestor.
- Loss of heterozygosity
-
(LOH). Allelic imbalance by which a heterozygous somatic cell becomes homozygous because one of the two alleles gets lost.
- Fixation
-
When a genetic alteration reaches 100% frequency in a cell population.
Rights and permissions
About this article
Cite this article
Schmidt, M., Hackett, R.J., Baker, AM. et al. Evolutionary dynamics in Barrett oesophagus: implications for surveillance, risk stratification and therapy. Nat Rev Gastroenterol Hepatol 19, 95–111 (2022). https://doi.org/10.1038/s41575-021-00531-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00531-4