Abstract
The yellow-legged gull Larus michahellis has undergone widespread colonization of the urban environment in the recent past. The first urban breeding gulls were recorded in the historical centre of Venice, Italy, in 2000, and by 2005 there were already 24 roof-nesting pairs, with this number increasing significantly over the last decade. In 2016, a new door-to-door garbage collection system was introduced in Venice to prevent the accumulation of rubbish in the streets and limit the trophic resources available for the species. This study provides an up-to-date estimate of the Venice yellow-legged gull urban population using distance sampling method. We also studied the effect of the new waste collection system on the species by comparing the population estimate before (2017) and after (2018) the full implementation of this change and by analysing the trend of individuals collected in the old town by the wildlife recovery service during 2010–2018. Results estimated ca. 430 breeding pairs in June 2018 showing a 36% decrease with respect to 2017. We also found a decrease in the number of 1-year-old birds and pulli collected by the wildlife recovery service starting from 2016, when the policy implementation began. Our data did not show a significant decrease in the overall number of individuals, suggesting that the new policy has a stronger effect on the breeding success of the species than on adult survival. This study emphasizes the importance of preventing rubbish accumulation in the streets as factor for reducing the abundance of urban yellow-legged gulls.
Similar content being viewed by others
Introduction
Over the centuries, and particularly from the beginning of the industrial revolution, humankind has profoundly transformed the Earth’s surface by converting the original landscape into anthropic ecosystems, mainly represented by urban areas and farm fields (Meyer and Turner 1992; Houghton 1994; Berry 2008), thus generating major pressures on other life forms and exerting selective pressures which drive the evolution of many species (Alberti et al. 2003; Ellis 2015; Albuquerque et al. 2018). As a whole, human actions and urban sprawl have led to a homogenization of landscapes by destroying and fragmenting the original habitats (McKinney 2006). This has resulted in a generalization of the wildlife communities (Marzluff 2001), with the loss of rare and specialized species (commonly known as “losers”) and an increase of generalist and adaptable ones (“winners”) (McKinney and Lockwood 1999; McKinney 2008). The latter species, defined as synanthropic (from the Greek syn + anthropos, “together with man”), have adapted to live in highly anthropized habitats, well tolerating the disturbance effects from anthropogenic pressure and activities (e.g., traffic noise, air, water and soil pollution) and indeed taking advantage of human presence (Rodewald and Shustack 2008).
Among birds, gulls (Larus spp.) have become so well adapted to the urban context that they are now superabundant and have started to be considered a pest species (Blokpoel and Spaans 1991; Feare 1991). In Britain and Ireland the herring gulls Larus argentatus and the lesser black-backed gulls Larus fuscus increased at a rate of 12–13% per annum from the beginning of the century to the mid-1970s (Raven and Coulson 1997; Coulson 2015) and the yellow-legged gull Larus michahellis showed a clear demographic growth in Italy, by increasing from 24,000–27,000 breeding pairs in 1983 (Meschini and Frugis 1993) to the 45,000–60,000 in the early 2000s (Brichetti and Fracasso 2006). Overall, this species has undergone a widespread population explosion over the past 30 years in the whole Mediterranean basin (Vidal et al. 1998).
Gulls’ demographic growth has been accompanied by a spread of the breeding range with the colonization of the urban environment, where individuals began to nest on the rooftops and terraces of buildings (Monaghan and Coulson 1977). In Britain and Ireland, the herring gulls and the lesser black-backed gulls began to nest on buildings in 1940 and spread dramatically till the mid-1970s, showing a mean increase rate of 17% and 28% per annum, respectively (Raven and Coulson 1997). Starting from 1970s the colonisation of urban areas by gulls affected other European countries (Spaans et al. 1991; Rock 2005, 2013). In France, the first urban-nesting gulls were recorded in 1970 (Cadiou 1997), while in Spain they were recorded in 1975 (Petit et al. 1986).
In Italy, the first urban colony of yellow-legged gull dates back to 1971 in Rome, but it was only since the early 1980s that the phenomenon of gulls nesting on roofs has increased and spread to other cities: Sanremo (1982), Livorno (1984), Genova (1986), Trieste (1987), Naples (1990), highlighting a rapidly growing trend (Fraissinet 2015). The increasing use of urban habitats by gulls has led to a series of human problems: from the acoustic nuisance, especially in the gulls’ reproductive period, to fouling and damaging of the architectural and monumental heritage, to the aggression of adults in defence of their chicks (Dwyer et al. 1996; Soldatini et al. 2008) and the conflicts with commercial premises such as fish markets, butchers, bars or street food vendors (Belant 1997; Serra et al. 2016). A detailed analysis of the economic costs associated with a gull species (i.e., silver gull Larus novaehollandiae) on the human community, as well as the mitigation of such conflicts, has been performed in the Greater Melbourne Area, Australia (Temby 2000, 2004).
In the historical city centre of Venice, Italy, the first individuals showing reproductive behaviour were observed in 2000. In 2005, 24 roof-nesting pairs were recorded in the whole urban area (Soldatini and Mainardi 2006), while the latest published estimate available for the species indicate 50 pairs (Bon and Stival 2013). However, over the last 25 years, the breeding population of yellow-legged gulls has increased dramatically in the lagoon surrounding the city of Venice, rising from an estimated average number of 1350 pairs in 1990–92 (Scarton 2017) to the 4803 in 2013–2015, primarily located in suitable anthropogenic habitats such as dredge islands and artificial saltmarshes (Scarton and Valle 2017). The demographic growth experienced by the species in the Venetian lagoon was followed by an increase in the number of individuals attending the historic centre of Venice both for breeding and feeding purposes, exacerbating the problems of coexistence with this species (Coccon et al. 2019). Among the main critical issues which have been widely documented by the local press are the recurring attacks on passers-by to steal their food and the breaking open of garbage bags dropped by residents and tourists on the street and spreading of their contents both on the ground and in the city's canals (Coccon and Fano 2020). The latter situation has created what appear to be open-air landfills in the city leading to a negative image of Venice, considered an iconic place all over the world. To counter such problems, the public waste management company of Venice (Veritas S.p.a.) established a new door-to-door garbage collection system and garbage self-disposal to temporary waste disposal stations located on boats moored in specific areas of the city. This was to prevent the accumulation of rubbish in the streets and limit the amount of trophic resources available for the yellow-legged gulls. With this regard, a recent study (Coccon and Fano 2020) revealed that the new urban waste collection regime had a significant effect on lowering both the presence of waste and gulls attending the city for feeding purposes. However, the effect of the new policy on yellow-legged gulls’ breeding population has not been investigated yet. To fill this gap, we started a monitoring program with the aim of providing an updated and accurate estimate of the species (i.e., number of individuals and breeding pairs) in the historic centre of Venice. This is urgently needed since data from the last monitoring of the species in the city date back to 2005 and are no longer usable to support decision-making concerning urban control and management of the species. To achieve this goal, we used a distance sampling approach (Buckland et al. 2001) applied to counts performed from elevated observation points. Distance sampling is a widely used method that uses the distances to individuals/clusters recorded by surveying line or point transects for estimating animal density or abundance, on the assumptions that every bird is detected at a zero distance and that the probability of detection decreases as distance from the observer increases (Buckland et al. 2001). In this case, we used point-transect sampling, instead of line-transects. We also investigated the initial effects of the new waste collection system on the yellow-legged gulls using two different approaches:
-
i.
by comparing the number of individuals and breeding pairs when the new waste collection policy affected only a part of the city, in 2017, when gulls still could find food in form of waste simply by moving from one district to another (hereafter the “before” phase), and when the new system was fully implemented throughout the city, in 2018 (hereafter the “after” phase). For the comparison we used data from two districts surveyed in both years 2017 and 2018;
-
ii.
by analysing the trend of individuals (i.e., pulli, juveniles and adults injured or in difficulty) collected in the urban area from 2010 to 2018 by the wildlife recovery service performed by the Metropolitan City of Venice, as we assumed this trend may be used as an index for the urban gulls’ population for those years in which monitoring was not conducted.
We expected that the estimates in 2018 would be noticeably lower than those obtained in 2017, especially concerning the number of breeding pairs, as an effect of the widespread reduction of food availability in 2018 following the implementation of the new waste collection system, as highlighted by Coccon and Fano (2020). Results from this study will contribute to updated and accurate knowledge of the situation of yellow-legged gulls in Venice and will provide useful information on whether or not the new waste collection policy has been successful in lowering the problem of urban gulls. Finally, our work emphasises the potential value of distance sampling to provide accurate estimates of population and the possibility of this method being applied in other urban contexts for monitoring programs and for managing the species in the medium and long term.
Methods
Study area
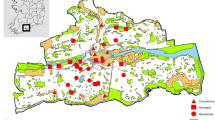
Our study has been conducted in the historic centre of Venice, Italy (45°26′13.67" N 12°19′57.54" E, 6.52 km2), which is divided into six districts (Fig. 1): Cannaregio (1.40 km2, 15,605 inhabitants), Castello (1.90 km2, 13,424 inhabitants), Dorsoduro (0.97 km2, 6430 inhabitants), Santa Croce (1.42 km2, 4939), San Polo (0.34 km2, 4612), San Marco (0.54 km2, 3750) (data of inhabitants updated 1st January 2017 provided by the Statistics and Research Office of the Municipality of Venice). With regards to the latter, despite the particularly low residential rate, there is the highest production of waste (amount of waste produced daily in the district) which is equal to 32 tons per day (data updated 31th December 2017 provided by the public waste management company of Venice, Veritas). Tourist pressure contributes significantly to this value, as demonstrated by the conspicuous number of tourist accommodation facilities officially declared to the Municipality of Venice here present (n = 199) (see Table S1).
Historic centre of Venice divided into six districts: Dorsoduro (DD), Santa Croce (SC), San Polo (SP), San Marco (SM), Cannaregio (CN) and Castello (CS). The observation points (numbered black dots) selected in each district for monitoring yellow-legged gulls are also depicted (see Table S2 for specific information on observation points). Venice is located in the Northeast of Italy
Data collection
The monitoring activity was performed from 16 observation points (OPs, bell towers or private and public panoramic terraces; Table S2) distributed in all the six above-mentioned districts (Fig. 1) and with a minimum distance of separation between them of 377 m. The number and distribution of OPs in each district depended on the availability of vantage points and on the extent and visibility (height of the nearby buildings relative to the OP height) recorded in the area. A pilot survey was carried out in 2017 focusing on Dorsoduro and Santa Croce districts (OP 1 to 8 in Fig. 1). In 2018 monitoring was extended to the whole historic centre of Venice, thus using all 16 OPs. In each OP we performed two monitoring sessions: in March, at the beginning of the reproductive season, when yellow-legged gulls start to occupy their reproductive sites and in June, when the breeding season reaches its climax and the probability of detecting juveniles near the nest is higher (Fracasso et al. 2011). The monitoring activity was performed in the morning, starting at dawn to detect the birds’ peak activity time and ending at most 3–4 h after dawn (Parra-Torres et al. 2020). This period was necessary in order to explore the roofs in detail and to detect the individuals. During surveys we recorded all the yellow-legged gulls detected while resting on the building roofs or on the ground, as well as those individuals that passed from flight to rest and vice versa. Birds in flight were not considered for the estimates. Data were collected by two professional ornithologists equipped with 10 × 42 binoculars (models: Leika Ultravid and Zeiss Terra ED 42) and one 20x-60 × spotting scope (Kowa TSN-883 Fluorite). In this way, while one observer was intent on determining the position of the yellow-legged gulls, the other tracked their movements in order to avoid the possibility of detection errors such as missing of individuals or double counts. The number of breeding pairs was also recorded. We considered a breeding pair to be two adults observed in a suitable nesting habitat, showing a territorial behaviour and/or performing alarm calls, or one individual while incubating eggs or bringing food to the chicks. Even the sole presence of chicks at the nest was considered to be an indicator of a breeding pair. For data collection we used an application for mobile devices, specifically developed for this study, which allowed us to record the spatial location and the characteristics of each observation (no. of individuals, presence of a breeding pair, a nest or chicks). This was possible by using Google Satellite as the basic map for recording the data, with the possibility of switching to the high-resolution georeferenced orthophotos of the historic centre of Venice, provided by the Venice Municipality, if the internet connection was not available. Data were stored in the device in real time and then exported to a Geographical Information System (ESRI, ArcGIS 10.2 for Desktop) platform for subsequent processing. Individuals detected in clusters were treated as single sightings, geolocating the centroid of the group. We considered a cluster when the individuals were located at a mutual distance less than 3 m.
Estimate of the abundance yellow-legged gulls’ breeding population
Point counts were analysed using distance sampling (Buckland et al. 2001, 2015) which has been suggested as an effective bird survey method in urban environment (Giunchi et al. 2007).
In order to obtain reliable estimates using distance sampling, four assumptions need to be satisfied:
-
Observation points need to be randomly distributed with respect to the species’ distribution. In our case the OPs were distributed opportunistically and not randomly, thus possibly leading to a biased estimate of population density. Given the peculiarity of the OPs, this bias could not be avoided, but it seems reasonable to assume that its effect on the abundance estimation should not be significant as the OPs was chosen independently from yellow-legged gull distribution.
-
All yellow-legged gulls located on or very near the OP should be detected. Due to the characteristics of the OP (see Table S2), it was sometimes impossible to see directly below them and thus a blind area existed exactly below the OP. For this reason, all data were left-truncated at 10 m from the OP.
-
Yellow-legged gulls were detected in their initial position, before being disturbed by the observer. To reduce this effect, monitoring started about 10 min after the observers had taken position to allow yellow-legged gulls to get used to them.
-
Distances were measured accurately. Thanks to the use of the mobile application for mapping all detected birds, distance from the OPs to each record were derived, using ArcGIS, with an estimated accuracy of ± 1 m. Distances were calculated by disregarding the height of the OPs and of the detected birds.
Distance data were analysed using the package Distance 1.0.1 (Miller et al. 2019) in the R 4.0.2 environment (R Core Team 2020). We performed two different types of analysis: in the first one we estimated the abundance of individuals, while in the second one we estimated that of breeding pairs, thus excluding all birds with no indication of reproductive activity (see description above). The data from each survey were analysed independently as the sample size was large enough (> 75 detections, Buckland et al. 2001) and preliminary data explorations indicated that the use of pooled data would not represent a significant improvement for the analysis (analysis not reported).
For the individual-based analysis we modelled the detection-probability function considering the clusters of individuals; density (number of individuals per km2) estimation was then calculated by multiplying clusters density by mean cluster size, since a preliminary check of the data did not indicate any size bias problem (Buckland et al. 2001). In the second analysis, the detection-probability function was modelled by considering one breeding pair as a single detection. In modelling the detection-probability function we considered four models (Thomas et al. 2010): half-normal key with cosine and Hermite polynomial adjustments, uniform key with cosine adjustments, and hazard-rate key with simple polynomial adjustments. To improve the fit, data were right-truncated at 500 m. As mentioned above, all data were also left-truncated at 10 m from the OP in order to take into consideration the blind area exactly below each OP. Mean cluster size was calculated using truncated data. For each OP the survey effort was obtained by considering the proportion of urbanized area included in the buffer calculated at the right truncation distance. In this way we excluded the area occupied by the water of the lagoon, which was not considered in the survey. Detection function models were ranked according to the Akaike Information Criterion, AIC (Buckland et al. 2001; Burnham and Anderson 2002) and their fit tested using the Cramér-von-Mises goodness of fit test. Estimated densities/abundances were obtained by means of model averaging using Akaike weights (wi) and considering models within two AIC units from the best candidate (Burnham and Anderson 2002). Model-averaged 95% confidence intervals (95% CI) were calculated according to Turek and Fletcher (2012), using the package RMark 2.2.7 (Collier and Laake 2013). Comparisons among estimates were performed by considering 95% CI, as suggested by Johnson (1999).
Analysis of the initial effects of the new waste collection system on gull population
The new waste collection system consists of door-to-door garbage collection from private households and commercial premises, approximately between 8.00 and 10.00 a.m. Garbage self-disposal is also available at the temporary and movable waste stations located on boats moored in specific areas of the city within a given time schedule between 6.30 and 8.30 a.m., preventing in this way that gulls have access to waste. This method has replaced the old one, which established that garbage bags were left by residents and tourists on the street, in front of their homes or businesses, for the ecological operator to collect between 6 and 8 a.m. However, garbage bags were often deposited on the street at times not suitable for collection by ecological operators, therefore gulls had plenty of time to break them and eat the leftovers, leading to problems of sanitary nature as well as of urban décor. The new policy was introduced in the districts of the historical city centre of Venice at different times. Specifically, starting from September 2015, it was applied on an experimental basis in a portion of Dorsoduro characterized by a low residential rate and then it was extended to the remaining part of this district in October 2016. The policy was then applied to Santa Croce and San Polo in March 2017, to San Marco in May and to Cannaregio in December. The last district was Castello, where the new method for collecting waste was introduced in May 2018 (Coccon and Fano 2020). Unfortunately, due to lack of funds, it was not possible to perform the monitoring of the urban yellow-legged gulls while the old waste collection system was still in place in all the city. Therefore, we decided to use data collected during the pilot survey of 2017 as representative of the “before” phase. In fact, in that period, the new waste collection system had been introduced in the city only partially and recently, specifically since October 2016 in Dorsoduro and since March 2017 in Santa Croce. This mixed situation had not created a real clear cut with the previous one, given the proximity between districts that allowed gulls to equally find food, by moving from one district to another. On the contrary, data collected in 2018 were considered as representative of the “after” phase, since the new policy was fully implemented throughout the historic centre. This situation profoundly changed the aspect of the city, drastically reducing the trophic availability for the species as demonstrated by the study of Coccon and Fano (2020). Hence, to evaluate the initial effects of the policy change we used two different proxies: i) we compared the density estimation of yellow-legged gulls in the two districts surveyed in both years 2017 and 2018 (i.e., Dorsoduro and Santa Croce); and ii) we analysed the trend of yellow-legged gulls collected in the Venice historical city centre from 2010 to 2018 by the wildlife recovery service, as we assumed it may reflect the trend of yellow-legged gulls’ urban population for those year in which monitoring had not been conducted. Indeed in a city like Venice, where you move by walking in the streets, it is particularly easy to notice if there are animals in difficulty, whether they are chicks, juveniles or adults, and it is possible to assume that most of them had been collected by the wildlife recovery service, both because of widespread interest of citizens in urban fauna, and efficiency of the service. Wildlife recovery data were provided by the Veneto Region—Direzione Agroambiente, Programmazione e Gestione ittica e faunistico venatoria-Unità Organizzativa Coordinamento gestione ittica e faunistico-venatoria Ambito Litoraneo- Sede Territoriale di Venezia.
Comparison of the density estimation before (2017) and after (2018) the change of the waste collection policy
Density estimates of individuals and breeding pairs in March and June 2017 and 2018 were obtained using the same approach as detailed above for population estimate, except for the use of the data belonging to only eight of the sixteen observation points (OPs from 1 to 8, Fig. 1), which covered the two districts surveyed in both years (i.e., Dorsoduro and Santa Croce). We considered density and not abundance since the estimates concerned a fraction of the study area.
Analyses of the trend of yellow-legged gulls collected in the Venice historical city centre by the wildlife recovery service from 2010 to 2018
The trend of yellow-legged gulls collected in the urban area by the wildlife recovery service from 2010 to 2018 was analysed by means of a Generalized Additive Model (GAM) with Poisson error structure and log link estimated with the package mgcv 1.8–33 (Wood 2017) in the R 4.0.2 environment (R Core Team 2020). The predictors of the model were the year and the age class of the collected bird (two-level factor: a) > 1-year-old birds; b) 1-year-old birds and pulli). The model included the main effects “Year” and the ‘smooth-factor’ interaction “Year by Age”, which produced a separate smooth for the two levels of Age (Wood 2017). We used a thin plate regression spline as smooth basis, setting k = 8 as the maximum basis dimension; the actual degree of smoothing was estimated by general cross-validation (Wood 2017). Model assumptions (autocorrelation and distribution of residuals, homogeneity of variance, influential observation, overdispersion) were checked according to Wood (2017) using the mgcv package.
Results
Estimate of the abundance of yellow-legged gulls’ breeding population
Excluding the survey performed in June, only considering the breeding pairs, the half-normal key with no adjustment was the highest-ranking model for the detection function in all the analyses (Table 1; Figs. S1 and S2). The fit of the models was good both for individuals and breeding pairs after a reasonable right-truncation which excluded ≤ 21.1% of observations from the analysis. According to the estimated detection probability, all models provided relatively comparable results in both the studied period.
Density estimates, obtained by means of model averaging, turned out to be quite precise in both months of the survey, with a coefficient of variation less than 0.20 in all cases (Table 2). The density of gulls was higher in March and then markedly decreased in June. According to our results the number of gulls in Venice varied on average between 2000 and 3000 individuals (Fig. 2). It is important to emphasize that this estimate probably represents a minimum abundance as we did not consider birds in flight in our estimation.
Estimated abundance ± 95% CI of individuals (left) and breeding pairs (right) of yellow-legged gulls in the historic centre of Venice in the considered months of 2018. Estimates were obtained by means of model averaging considering models within two AIC units from the best candidate (see Table 1)
The density and estimated number of breeding birds turned out to be less than a quarter of the estimated number of individuals (Table 2; Fig. 2). The relative decrease of abundance recorded between March and June was slightly more pronounced for breeding pairs (–34%, on average: 655 vs. 430), than for individuals (–25%, on average: 2786 vs. 2082). However, given the overlap of 95% CI, these estimates were not significantly different.
Analysis of the initial effects of the new waste collection system on gull population
Comparison of the density estimation before (2017) and after (2018) the change of the waste collection policy
The fit of the models selected for the detection function of surveys performed in March and June of the years 2017 and 2018 from the selected eight Observation Points (OPs) was good both for individuals and breeding pairs after right-truncation (Table 3; Figs. S3 and S4). However, as expected given the low number of OPs used in the analysis, the precision of density estimates obtained by means of model averaging was not very high (Table 4). In both years, the density of individuals and breeding pairs tended to be higher in March than in June (Table 4; Fig. 3) with the estimate of individuals being quite comparable in the two years, while that of breeding pairs markedly lower in 2018, both for March and June (Fig. 3). These differences however were not statistically significant as the standard errors of the estimates were quite large and the 95% CI mostly overlapped. Nevertheless, the decrease observed in June is noticeable, with the density recorded in 2018 corresponding to about 64% of that recorded in 2017.
Estimated density ± 95% CI of individuals (above) and breeding pairs (below) of yellow-legged gulls in Dorsoduro and Santa Croce when the waste collection policy change affected only a part of the city (i.e., March and June 2017, representative of the “before” phase) and when it was implemented in all the districts (i.e., March and June 2018, representative of the “after” phase). Estimates were obtained by means of model averaging considering models within two AIC units from the best candidate (see Table 3)
Analyses of the trend of yellow-legged gulls collected in Venice historical city centre by the wildlife recovery service from 2010 to 2018
The GAM model fitting the trend of the number of yellow-legged gulls collected in the urban area by the wildlife recovery service from 2010 to 2018 explained a large proportion of the deviance of the data (deviance explained = 92.2%, adjusted R2 = 0.90). As depicted in Fig. 4, the two age classes considered for the analyses show a different trend, especially in the last three years. The number of > 1-year-old birds increased over the sampling period at an increasing rate, while the number of 1-year-old birds together with pulli reached the peak in 2016 and then decreased markedly. In both cases, the fitted smooths turned out to be highly significant (> 1-year-old birds: edf = 2.02, χ2 = 70.7, p < < 0.001; 1-year-old birds + pulli: edf = 3.52, χ2 = 36.2, p < < 0.001).
Number of yellow-legged gulls collected in Venice historical city centre by the wildlife recovery service in the period 2010–2018. The fitted lines of the Generalized Additive Model with Poisson error distribution and logarithm as link function is also depicted for the two age classes considered (shaded area = 95% CI)
Discussion
In this work we provided an updated estimate of the yellow-legged gull population in the historic centre of Venice and investigated the initial effects of the new waste collection system on the species by comparing the abundance of individuals and breeding pairs when the policy change affected only a part of the city and when it was implemented in all the districts, as well as by analysing the trend of yellow-legged gulls collected in the city centre by the wildlife recovery service over an eight-year period.
Estimate of the abundance of yellow-legged gulls’ breeding population
For the estimation of the urban gulls’ population we used the distance sampling method (Buckland et al. 2001). To our knowledge, only one published study used this method for monitoring yellow-legged gulls in an urban environment (Bellout et al. 2021), with most using direct counts from vantage points and from street surveys (i.e., surveying rooftops from ground level) (Coulson and Coulson 2015) or conducing independently ground-based and aerial surveys (Rock 2002; Durham 2003; Sellers and Shackleton 2011; see Ross et al. 2016 for a review). However, it has been proved that direct counts miss an appreciable number of nests, with an average maximum nest detection rates of 78% for vantage point surveys and 48% for street ones (Coulson and Coulson 2015), while aerial surveys lead to some issues in distinguishing between gull species (Durham 2003; Sellers and Shackleton 2011). Recently, unmanned aerial vehicles (UAVs, or drones) have been used to census the urban-nesting gull population in the city of Victoria, Canada, being an effective technique in readily discerning the occupied nests and incubating birds which were not disturbed by the drones (Blight et al. 2019). However, flight restrictions for UAVs may render their use impracticable in several urban settings (ENAC 2020).
The distance sampling method has been successfully used in natural context to census breeding seabirds (Lawton et al. 2006; Kirkwood et al. 2007; Robertson et al. 2008) and its performance in estimating the total number of nests of a natural colony of large gulls has been compared with the strip transect counts method by Barbraud et al. (2014). Such study strongly advocates the use of distance sampling since estimates obtained by the strip transect count method were significantly lower (from 9 to 31%). Moreover, these authors promote the use of distance sampling as it creates less disturbance than transect counts and it requires fewer observers and less time than direct counting methods (Barbraud et al. 2014). Therefore, it is reasonable to assume that distance sampling is even more recommendable in urban environments, the latter being more complex than natural settings and with a higher risk of bias (Giunchi et al. 2007). A potential issue related to the use of distance sampling in urban habitats is the high number of visual hindrances which determines a spiked distribution of distance data (Giunchi et al. 2007; Bellout et al. 2021), which is often difficult to model (Buckland et al. 2001). In the present study, the use of elevated vantage points overcomes this problem, allowing for a more robust modelling process and a consequent reduction in potential biases.
Density estimates of yellow-legged gull urban population in 2018 actually turned out to be quite reliable, given the precision and the consistency of the estimated detection probability among different models, providing evidence that the number of urban breeders in Venice correspond approximately to the 41% of the estimated individuals. According to previous studies (Kadlec and Drury 1968; Coulson et al. 1982), 15 to 30% of the population of large gulls’ species comprise non-breeding adults. In this case, the fraction seems higher (around 59%) but it is possible that the overall number of estimated individuals was biased low as flying birds were not included in the counts.
Obtained estimates are rather different from those of 2005, when the urban colony was at the beginning of its growth and probably involved relatively young breeders (Soldatini et al. 2008), showing an average annual increase rate of about 25% between 2005 and 2018.Trends similar to that shown by yellow-legged gulls in the historical centre of Venice have also been found in other urban areas, both in Italy (Benussi and Fraissinet 2020) and abroad (Rock 2013; Ross et al. 2016). As an example, in Britain and Ireland, large gulls breeding on rooftops showed an annual average increase rate of 17% for herring gulls and 28% for lesser black-backed gulls between 1969 to 1976 (Monaghan and Coulson 1977) and, despite the national decline highlighted by the herring gulls in Britain since about 1970, both the species continued to increase and spread in the cities (Raven and Coulson 1997; Coulson 2015), to reach in 1998–2002 the 20,000 roof-nesting pairs of herring gulls and the 11,000 pairs of lesser black-backed gulls, which is respectively more than double and four times the number recorded in 1994 (Mitchell et al. 2004). Estimates, however, are often obtained with different methods, posing a problem of comparability and different degrees of reliability of data.
Several factors have led to the colonization of urban areas by large gulls. First of all, it has to be mentioned the strong recovery experienced by many seabirds during the twentieth century after the decline of the nineteenth century due to their persecution (Coulson 1963), and the consequent need of finding new sites suitable for breeding. Regarding this, buildings in towns are sort of man-made islands within a “sea” of concrete that show many of the attributes afforded by natural breeding sites, as reported by Coulson and Coulson (2009). In the case of the Venice historical city centre, there are some peculiarities that make it particularly attractive to gulls, such as the massive presence of historical buildings and churches (n = 133 in the studied area, according to data provided by the Territorial Information Systems Service Office of the Venice municipality), all characterized by tiled roofs that make them inaccessible to people and therefore freely usable by yellow-legged gulls for nesting and resting purposes. Another characteristic of the city is the presence of a large amount of street food, bars and restaurants providing take-away food that have exploded in the city in recent years to satisfy the ever-increasing demand for “hit and run” tourism. The old town of Venice records over 10 million tourist presences every year to which are added the “visitor” tourists (i.e., people who do not spend the night in the city), whose number is uncertain but, in any case, greater than a further 12 million/year (Campostrini and Dabalà 2017). The latter generally consume quick and cheap meals while strolling around the city, in this way attracting yellow-legged gulls that are waiting to steal their food or to eat the leftovers abandoned on the street (Coccon F. pers. obs.). Last, but not least, the city of Venice is surrounded by its lagoon that is the widest of the Mediterranean area, covering an area of 55,000 ha, listed as Important Bird Area since 1989 (Heath et al. 2000) and recognised as a Special Protection Area (SPA IT 3250046 Lagoon of Venice) since 2007, according to the 147/2009 Birds Directive of the European Union. This synergy allows yellow-legged gulls to exploit either the resources of anthropogenic origin found in the city or the widespread natural sources offered by the lagoon for both nesting and feeding purposes. With regards to the latter, according to a recent study (Spelt et al. 2019) urban-nesting gulls in Bristol (UK) spend on average two-thirds of their time away from the nest in the suburban and urban areas and one-third in rural green areas such as agricultural fields, while almost completely avoiding the marine areas close to the city, suggesting in this way that for these urban-nesting birds the net energy gain of foraging in the available terrestrial environment (e.g., human refuse, earthworms and insects) is higher than for the local marine environment. However, in our case, information on the movements of urban nesting gulls outside the city are not available, therefore we can only assume that, as a result of the decrease in trophic resources in the city following the waste collection policy change, gulls started to increase the exploitation of the mainland and lagoon resources, looking for food.
Importantly, the trend shown by the species in the Venice historical city centre is in contrast with that found in natural and artificial habitats (e.g., littoral strips, natural and artificial salt marshes, dredge islands, fish farms, restored habitats) of the north-eastern Adriatic coastline, where a moderate but statistically significant decline of the species has been recorded in the period 2008–2014 (Scarton et al. 2018). Additionally, if considering yellow-legged gulls breeding in the Venice lagoon, where most of nests are located in dredge islands and artificial salt marshes (Scarton and Valle 2017), an average annual increase rate of 1.3% was found between 2010 to 2018 (Scarton F. unpublished data), thus a fairly low value with respect to the one recorded in the old town. These findings are in line with those shown in previous work conducted on the west coast of Canada (Vermeer 1992), which found a higher annual increase rate of breeding glaucous-winged gulls Larus glaucescens in downtown Vancouver (9% yearly increase of roofs utilised by gulls and of number of gulls nesting on roofs), compared with that of the Strait of Georgia, where the average annual increase rate over the period 1975–86 was 2.6%. It is likely that the higher annual growth rate recorded in urban environment is linked to the higher reproductive success due to the lower predation rate than in natural habitats (Monaghan 1979; Coulson and Coulson 2009), besides the larger availability of food, in the form of domestic and commercial waste and leftovers, which are easily reachable also by inexperienced juveniles (Luniak 2004).
Our results show a decreasing trend of the yellow-legged gulls’ abundance from March to June. This finding is in line with that found in Rome, Italy, where the sharp decline recorded by the urban population of the species in summer months has been interpreted as an abandonment of the city in favour of searching for food in other sites, following the significant decrease of people staying in the city during holidays and therefore of trophic resources available to gulls (Fraticelli 2017). However, this explanation is not plausible for the city of Venice, where the tourism rate is high all over the year and actually increases in the summer period (Città di Venezia 2018). In this case, the decrease of the yellow-legged gull urban population observed in June is probably linked to the abandonment of the city by those individuals that have failed their breeding attempt(s) and thus move elsewhere in search of attractive sources, present both in the lagoon and on the mainland (Coccon et al. 2015), to reduce intraspecific competition within the city.
Analysis of the initial effects of the new waste collection system on gull population
Our study did not show any decrease of individuals between 2017 and 2018 but highlighted a decrease of breeding pairs which was particularly evident in June (–36%). Such a decrease is not statistically significant, probably because of the small number of observation points (n = 8) used for the comparison of the population between the two years that leads to a low precision of the estimates. This limitation, however, is unsurpassable since for 2017 (i.e., the year representative of the “before” phase) we had data available for two districts only. Anyway, this result is particularly encouraging as it predicts a decline of new-born gulls in the city. It also strengthens the thesis supported by Belant (1997) that limiting availability of food, in the case of Venice by preventing the rubbish accumulation in the street, is a key element in controlling the urban population of the species since it reduces the overall suitability of the area for gulls. These findings agree with previous work (Pons 1992) according to which a reduction in trophic availability tends to have a much more dramatic effect on breeding success of the species than it does on adult survival, as adults are able to adapt and find other forms of sustenance (Pierotti and Annett 1991). In the case of Venetian gulls, following the policy change, they started to forage on crabs and cuttlefish in the city canals, they intensified predation on rock-doves and swifts, as well as kleptoparasitism on the food of passers-by and served in bars and restaurants, even begging citizens and tourists (Coccon and Fano 2020). Interestingly, results provided by distance sampling are in line with those obtained by analysis of data from the wildlife recovery service. Indeed, the number of pulli and 1-year-old birds recovered showed a clear decline after 2016, i.e., the year of implementation of the new waste collection system in a portion of the city. It is possible that this trend is actually due to a decrease of breeding pairs, as suggested by distance sampling, which led to a reduction in the number of chicks born, and to limited food availability following the policy change (Coccon and Fano 2020), which possibly forced juveniles to move elsewhere in search of new foraging areas, as competition for trophic resources probably increased in the city. On the contrary, the number of recovered > 1-year-old birds showed a noticeable increase after 2016. It might be supposed that this increase is due to the enhanced competition for limited resources that may have led animals to fight for grabbing food, therefore injuring themselves (Coccon F. pers. obs.), or to ingest worse quality food or even starving, with consequent higher probability of finding them in difficulty.
To sum up, the available data suggest that the new waste management policy affected not only the number of foraging yellow-legged gulls, as reported by Coccon and Fano (2020), but also the abundance of breeding pairs and possibly their breeding success, even though our data does not provide a clear-cut demonstration of this effect. The short period (i.e., two years) considered in this analysis does not allow us to exclude the possibility that the observed variability in gulls’ numbers could be affected, at least in part, from factors others than waste availability (e.g., food availability in the lagoon). Repeated surveys in the coming years, possibly integrated with GPS tracking data (see Spelt et al. 2019) on urban-nesting individuals, will allow a clearer understanding of the impact of the waste management on the yellow-legged gulls’ breeding population in Venice.
Conclusions
In this study, we updated the estimate of the urban population of yellow-legged gulls for the historic centre of Venice using distance sampling (Buckland et al. 2001) applied to counts performed from elevated vantage points, a novel monitoring method for the species in urban environment. This method provided a reasonable estimate of population size, outlining a picture very far from the last one described for the area by Soldatini and Mainardi (2006). This underlines the importance of long-term monitoring, to be carried out possibly continuously or at least every 2–3 years, to detect numerical and behavioural changes in the species and provide an updated picture of the urban population in order to properly manage it. There are, however, some intrinsic limits to the estimates obtained in this work. In fact, it is important to note that the estimate of individual abundance is probably biased low as we did not consider birds in flight. Future studies should be focused on evaluating the long-term effects of the new waste collection policy on yellow-legged gull urban population. Density and abundance estimates may be improved by considering both roosting and flying animals (Bibby et al. 2000). Finally, it would be interesting to study the effects on the target species of measures to combat and contain the Covid-19 pandemic which has profoundly changed the city, by emptying it of tourists and daily visitors. This would indirectly provide information on the influence of tourism on the presence and distribution of the species in the city.
Data availability
Data and materials are available upon reasonable request to the authors.
References
Alberti M, Marzluff JM, Shulenberger E, Bradley G, Ryan C, Zumbrunnen C (2003) Integrating humans into ecology: Opportunities and challenges for studying urban ecosystems. Bioscience 53(12):1169–1179
Albuquerque UP, Gonçalves PHS, Júnior WSF, Chaves LS, da Silva Oliveira RC, da Silva TLL, dos Santos GC, de Lima AE (2018) Humans as niche constructors: Revisiting the concept of chronic anthropogenic disturbances in ecology. Perspect Ecol Conserv 16(1):1–11
Barbraud C, Fortin M, Charbonnier Y, Delord K, Gadennne H, Thiebot J-B, Gélinaud G (2014) A comparison of direct and distance sampling methods to estimate abundance of nesting gulls. Ardeola 61(2):367–377
Belant JL (1997) Gulls in urban environments: Landscape-level management to reduce the conflict. Landsc Urban Plan 38:245–258
Bellout S, Baamrane MAA, Aamiri A, Aourir M (2021) Changes in the population size of yellow-legged Gull Larus michahellis at Essaouira and Mogador Island, west-central Morocco. Mar Ornithol 49:101–107
Benussi E, Fraissinet M (2020) The colonization of the Western yellow-legged gull (Larus michahellis) in an Italian city: Evolution and management of the phenomenon. Problematic Wildlife II, Springer, Berlin, p 191–212
Berry BJ (2008) Urbanization. Urban Ecology, Springer, Berlin, p 25–48
Bibby CJ, Burgess ND, Hill DA, Hillis DM, Mustoe S (2000) Bird census techniques. Elsevier, Amsterdam
Blight LK, Bertram DF, Kroc E (2019) Evaluating UAV-based techniques to census an urban-nesting gull population on Canada’s Pacific coast. J Unmanned Veh Syst 7(4):312–324
Blokpoel H, Spaans A (1991) Introductory remarks: Superabundance in gulls: Causes, problems and solutions. Acta Congr Int Ornithol 20:2361–2364
Bon M, Stival E (2013) Uccelli di laguna e di città. L’atlante ornitologico nel comune di Venezia 2006–2011. Marsilio Editore, Venezia
Brichetti P, Fracasso G (2006) Ornitologia Italiana, vol 3 (Stercorariidae-Caprimulgidae), Alberto Perdisa Editore, Bologna
Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L (2001) Introduction to distance sampling: Estimating abundance of biological populations. Oxford University Press, Oxford
Buckland ST, Rexstad EA, Marques TA, Oedekoven CS (2015) Distance sampling: Methods and applications. Springer, Berlin
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn. Springer Verlag, Berlin
Cadiou B (1997) La reproduction des Goélands en milieu urbain: Historique et situation actuelle en France. Alauda 65(3):209–227
Campostrini P, Dabalà C (2017) Una visione olistica, multidisciplinare ed integrata per il Piano di monitoraggio della costruzione del MOSE. In: Campostrini P, Dabalà C, Del Negro P, Tosi L (eds), Il controllo ambientale della costruzione del MOSE. 10 anni di monitoraggi tra mare e laguna di Venezia. CORILA, Venezia, p 13–35
Città di Venezia (2018) Annuario del turismo 2017, assessorato al turismo. Comune di Venezia, Centro Produzione Multimediale, Venezia
Coccon F, Fano S (2020) Effects of a new waste collection policy on the population of yellow-legged gulls, Larus michahellis, in the historic centre of Venice (Italy). Eur J Wildl Res 66(4):50. https://doi.org/10.1007/s10344-020-01384-z
Coccon F, Panzarin L, Dabalà C, Fano S, Vanni L, Giunchi D (2019) Gli effetti di una nuova politica gestionale dei rifiuti sulla popolazione di gabbiano reale a Venezia. In: Libro degli abstract-XX Convegno italiano di ornitologia, Napoli
Coccon F, Zucchetta M, Bossi G, Borrotti M, Torricelli P, Franzoi P (2015) A land-use perspective for birdstrike risk assessment: the attraction risk index. PLoS One 10(6). https://doi.org/10.1371/journal.pone.0128363
Collier BA, Laake JL (2013) RMark: An R interface to capture-recapture analysis with MARK. Mark Workshop Notes, Ft. Collins
Coulson J, Coulson B (2009) Ecology and colonial structure of large gulls in an urban colony: Investigations and management at Dumfries, SW Scotland. Waterbirds 32(1):1–15
Coulson J, Coulson B (2015) The accuracy of urban nesting gull censuses. Bird Study 62(2):170–176
Coulson J, Duncan N, Thomas C (1982) Changes in the breeding biology of the herring gull (Larus argentatus) induced by reduction in the size and density of the colony. J Anim Ecol 739–756
Coulson JC (1963) The status of the Kittiwake in the British Isles. Bird Study 10:147–179
Coulson JC (2015) Re-evaluation of the role of landfills and culling in the historic changes in the Herring Gull (Larus argentatus) population in Great Britain. Waterbirds 38(4):339–354
Durham M (2003) A survey of breeding gulls in and around Gloucester City. Severn Estuary Gull Group Bullet 14:11–15
Dwyer CP, Belant JL, Dolbeer RA (1996) Distribution and abundance of roof-nesting gulls in the Great Lakes region of the United States. Ohio J Sci 96(1):9–12
Ellis EC (2015) Ecology in an anthropogenic biosphere. Ecol Monogr 85(3):287–331
ENAC (2020) Regolamento mezzi aerei a pilotaggio Remoto - Edizione 3, Emendamento 1 del 14 luglio 2020
Feare CJ (1991) Control of bird pest populations. In: Peris CM, Lebreton JD, Hirons GJM (eds) Bird Population Studies. Oxford University Press, Oxford, pp 463–478
Fracasso G, Bon M, Scarton F, Mezzavilla F (2011) Calendario riproduttivo dell’avifauna nella regione Veneto. Associazione Faunisti Veneti, Venezia
Fraissinet M (2015) La colonizzazione dei centri urbani italiani da parte del Gabbiano reale (Larus michahellis) Conoscere il fenomeno, prevenirlo, gestirlo. ANCI e Assessorato all’Ambiente del Comune di Napoli eds, Napoli
Fraticelli F (2017) il monitoraggio delle popolazioni urbane di gabbiano reale. Ecol Urbana 29(2):41–44
Giunchi D, Gaggini V, Baldaccini NE (2007) Distance sampling as an effective method for monitoring feral pigeon (Columba livia f. domestica) urban populations. Urban Ecosyst 10(4):397–412
Heath MF, Evans MI, Hoccom D, Payne A, Peet N (2000) Important bird areas in Europe: Priority sites for conservation. 2 Volume Set. BirdLife, Cambridge
Houghton RA (1994) The worldwide extent of land-use change. Bioscience 44(5):305–313
Johnson DH (1999) The insignificance of statistical significance testing. J Wildl Manag 63(3):763–772
Kadlec JA, Drury WH (1968) Structure of the New England herring gull population. Ecology 49(4):644–676
Kirkwood R, Lawton K, Moreno C, Valencia J, Schlatter R, Robertson G (2007) Estimates of southern rockhopper and macaroni penguin numbers at the Ildefonso and Diego Ramírez Archipelagos, Chile, using quadrat and distance-sampling techniques. Waterbirds 30(2):259–267
Lawton K, Robertson G, Kirkwood R, Valencia J, Schlatter R, Smith D (2006) An estimate of population sizes of burrowing seabirds at the Diego Ramirez archipelago, Chile, using distance sampling and burrow-scoping. Polar Biol 29(3):229–238
Luniak M (2004) Synurbization-adaptation of animal wildlife to urban development. In: Shaw et al (eds) Proceedings 4th International Urban Wildlife Symposium. Tucson, p 50–55
Marzluff JM (2001) Worldwide urbanization and its effects on birds. Avian ecology and conservation in an urbanizing world. Springer, Berlin, pp 19–47
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Cons 127(3):247–260
McKinney ML (2008) Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst 11(2):161–176
McKinney ML, Lockwood JL (1999) Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14(11):450–453
Meschini E, Frugis S (1993) Atlante degli uccelli nidificanti in Italia. Suppl. alle Ricerche di biologia della selvaggina/Ins. naz. per la fauna selvatica XX, Gangemi, Roma
Meyer WB, Turner BL (1992) Human population growth and global land-use/cover change. Annu Rev Ecol Syst 23(1):39–61
Miller D, Rexstad E, Thomas L, Marshall L, Laake JL (2019) Distance sampling in R. J Stat Softw 89:1–28
Mitchell PI, Newton SF, Ratcliffe N, Dunn TE (2004) Seabird Populations of Britain and Ireland. JNCC, Peterborough
Monaghan P (1979) Aspects of the breeding biology of Herring-Gulls Larus argentatus in urban colonies. Ibis 121(4):475–481
Monaghan P, Coulson JC (1977) Status of large gulls nesting on buildings. Bird Study 24(2):89–104
Parra-Torres Y, Ramírez F, Afán I, Aguzzi J, Bouten W, Forero MG, Navarro J (2020) Behavioral rhythms of an opportunistic predator living in anthropogenic landscapes. Mov Ecol 8(1):1–8
Petit JG, Gabernet MEM, Gimeno JT, Gallisà EC (1986) Urban nesting of yellow-legged gulls in Barcelona (Spain). Mediterranean Marine Avifauna. Springer, Berlin, pp 509–511
Pierotti R, Annett CA (1991) Diet choice in the Herring Gull: Constraints imposed by reproductive and ecological factors. Ecology 72(1):319–328
Pons J (1992) Effects of changes in the availability of human refuse on breeding parameters in a herring gull. Ardea 80:143–150
R Core Team (2020) R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/index.html
Raven SJ, Coulson JC (1997) The distribution and abundance of Larus gulls nesting on buildings in Britain and Ireland. Bird Study 44(1):13–34
Robertson G, Moreno CA, Lawton K, Kirkwood R, Valencia J (2008) Comparison of census methods for black-browed albatrosses breeding at the Ildefonso Archipelago. Chile Polar Biol 31(2):153–162
Rock P (2002) Roof-Nesting Gulls in Gloucester. Survey conducted in May 2002. Report to the Planning Department, Gloucester City Council
Rock P (2005) Urban gulls: Problems and solutions. British Birds 98:338–355
Rock P (2013) Urban gulls. Why current control methods always fail. Rivista Italiana di Ornitologia 82(1–2):58–65
Rodewald AD, Shustack DP (2008) Consumer resource matching in urbanizing landscapes: Are synanthropic species over-matching. Ecology 89(2):515–521
Ross KE, Balmer DE, Humphreys E, Austin G, Goddard B, Rehfisch M (2016) Urban Breeding Gull Surveys: A Review of Methods and Options for Survey Design. BTO Research Report No. 680, The British Trust for Ornithology, The Nunnery, Thetford, Norfolk, p 58
Scarton F (2017) Long-term trend of the waterbird community breeding in a heavily man-modified coastal lagoon: The case of the Important Bird Area “Lagoon of Venice.” J Coast Conserv 21(1):35–45
Scarton F, Valle R (2017) Andamento recente (2013–2015) delle popolazioni di uccelli acquatici nidificanti nella laguna aperta di Venezia. Bollettino Del Museo Di Storia Naturale Di Venezia 67:113–123
Scarton F, Verza E, Guzzon C, Utmar P, Sgorlon G, Valle R (2018) Laro-limicoli (Charadriiformes) nidificanti nel litorale Nord Adriatico (Veneto e Friuli Venezia Giulia) nel periodo 2008–2014: consistenza, trend e problematiche di conservazione. RIO-Research in Ornithology 88(2):33–41
Sellers R, Shackleton D (2011) Numbers, distribution and population trends of large gulls breeding in Cumbria, northwest England. Seabirds 24:90–102
Serra L, Andreotti A, Kirov D, Nardelli R, Nissardi S, Pirrello S, Popov D, Sadoul N, Volponi S, Zucca C (2016) Guidelines for management of the breeding populations of the yellow-legged gull Larus michahellis in the saltpans and coastal wetlands of the Mediterranean (Linee guida per la gestione delle popolazioni nidificanti di Gabbiano reale Larus michahellis nelle saline e nelle zone umide costiere del Mediterraneo). ISPRA, Manuali e linee guida, 144/2016, ISPRA-Settore Editoria, Roma
Soldatini C, Albores-Barajas YV, Mainardi D, Monaghan P (2008) Roof nesting by gulls for better or worse? Ital J Zool 75(3):295–303
Soldatini C, Mainardi D (2006) Gabbiani a Venezia: Splendidi uccelli in una splendida città? Alula XIII 1–2:181–188
Spaans AL, Coulson JC, Migot P, Monaghan P, Pruter J, Vauk G (1991) The herring gull in north-west Europe. Acta XX Congressus Internationalis Ornithologici, Christchurch, p 2365–2371
Spelt A, Williamson C, Shamoun-Baranes J, Shepard E, Rock P, Windsor S (2019) Habitat use of urban-nesting lesser black-backed gulls during the breeding season. Sci Rep 9(1):1–11
Temby ID (2000) Pieces of silver: examples of the economic impact and management of the silver gull (Larus novaehollandiae) in Melbourne, Australia. In: Human Conflicts with Wildlife: Economic Considerations 19. https://digitalcommons.unl.edu/nwrchumanconflicts/19
Temby ID (2004) Silver Gulls: Urban waste creates flying problems. In: Lunney D, Burgin S (eds) Urban Wildlife: More than meets the eye. p 151–158
Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, Bishop JRB, Marques TA, Burnham KP (2010) Distance software: Design and analysis of distance sampling surveys for estimating population size. J Appl Ecol 47:5–14
Turek D, Fletcher D (2012) Model-averaged Wald confidence intervals. Comput Stat Data Anal 56(9):2809–2815
Vermeer K (1992) Population-growth of the Glaucous-Winged Gull, Larus glaucescens, in the Strait of Georgia, British-Columbia, Canada. Ardea 80(1):181–185
Vidal E, Medail F, Tatoni T (1998) Is the yellow-legged gull a superabundant bird species in the Mediterranean? Impact on fauna and flora, conservation measures and research priorities. Biodivers Conserv (7):1013–1026
Wood SN (2017) Generalized additive models: An introduction with R. Chapman and Hall/CRC Press, London
Acknowledgements
We thank L. Panzarin for assistance in data collection and the Venice Municipalized Company Veritas Spa for financially supporting the project. We are particularly grateful to AVM S.p.A. – Azienda Veneziana della Mobilità, T Fondaco Dei Tedeschi by DFS, Venezia Terminal Passeggeri S.p.A. and the priestly Church of Venice for the opportunity of access to bell towers and the other elevated observation points used for monitoring. We also thank three anonymous referees whose contribution has been determining in improving a previous version of the manuscript. A special thank goes to R. C. Jones for reviewing and improving the English language.
Funding
This study was funded by the Venice Municipalized Company Veritas S.p.A (Funding agreement VERITAS-CORILA n. 06/17/AC_71 and n. 20/18/AR01).
Author information
Authors and Affiliations
Contributions
Francesca Coccon and Dimitri Giunchi conceived and designed the study. Data collection and analysis were performed by Francesca Coccon, Lorenzo Vanni and Dimitri Giunchi. All authors contributed to write the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coccon, F., Vanni, L., Dabalà, C. et al. The abundance of yellow-legged gulls Larus michahellis breeding in the historic centre of Venice, Italy and the initial effects of the new waste collection policy on the population. Urban Ecosyst 25, 643–656 (2022). https://doi.org/10.1007/s11252-021-01175-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-021-01175-7