Abstract
The incidence of bariatric surgery is increasing due to obesity being one of our top public health challenges. As such, bariatric-related ophthalmic changes are a potentially new clinical area of knowledge, with increasing published evidence on post-bariatric complications experienced by patients and identified by clinicians. We reviewed the available literature and summarised the different complications and potential recommendations. A search strategy was conducted with PubMed, Cochrane, Medline, Embase, Allied and Complementary Medicine and DH-DATA databases to look for papers answering our research question: “What are the ophthalmological complications for patients after bariatric surgery?”. Our search gave a total of 59 relevant papers. Bariatric surgery, particularly subtypes that cause direct bypass of nutrients from the stomach, lead to nutritional deficiencies. Vitamin A, crucial for proper functioning of body systems and specialised cells, manifests ophthalmologically as corneal ulceration, nyctalopia, conjunctival xerosis and more. Thiamine levels are also depleted, leading to Wernicke’s Encephalopathy. Pre-existing diabetic retinopathy is also noted to worsen sub acutely, although evidence is conflicting. Patients undergoing surgery to treat idiopathic intracranial hypertension would have reduced IOP and resolving papilloedema. Other comorbidities of obesity like HBA1C levels, obstructive sleep apnoea, and metabolic syndrome also resolve post-surgery. History taking remains the cornerstone of medical practice. From the evidence, we suggest consideration of pre-surgery screening for ophthalmic pathology and post-operative monitoring of disease progression. Real-world data needs to continuously be analysed to create definitive management pathways that can help clinicians recognise ophthalmic complications early, improving patient outcomes.
摘要
肥胖是我们最大的公共卫生挑战之一, 减肥手术的实施率正在增加。随着关于减肥后并发症报道越来越多, 且这些都是由患者亲身经历或者经临床医生确认的, 因此与肥胖相关的眼部变化是一个潜在的新的临床知识领域。我们回顾了现有的文献, 总结了不同的并发症和潜在的建议。我们通过PubMed、Cochrane、Medline、Embase、联合和补充医学以及DH-DATA数据库进行了搜索, 以寻找能够回答我们关于““减肥手术后患者的眼科并发症是什么? ”问题的论文。我们共搜索到59篇相关论文。减肥手术, 特别是导致直接从胃中摄取营养的亚型手术, 会导致营养不足。维生素A对维持身体系统和特殊细胞的正常功能至关重要, 当维生素A缺乏时在眼部的表现为角膜溃疡、夜视、结膜干燥等。硫胺素水平也会下降, 并导致韦尼克脑病。术前存在的糖尿病视网膜病变也会亚急性恶化, 尽管目前的论文证据还存在相互矛盾。特发性颅内高压的患者在接受手术治疗后眼压会降低并缓解视乳头水肿。其它与肥胖的共患疾病如高糖化血红蛋白水平、阻塞性睡眠呼吸暂停综合症和代谢综合征也会在术后得到解决。病史记录仍然是医学实践的基石。根据证据, 我们建议考虑术前进行眼科病理筛查和术后的疾病进展监测。我们需要不断分析真实世界数据, 创建明确的管理途径, 帮助临床医生尽早识别眼部并发症, 改善患者预后。
Similar content being viewed by others
Introduction
Obesity, defined by the World Health Organisation (WHO) as an abnormal or excessive fat accumulation that poses a risk to one’s health [1], is one of the most concerning public health issues that is progressively overwhelming the global population. According to WHO statistics, from 1975 to 2016, the prevalence of overweight or obese children and adolescents aged 5–19 years increased more than four-fold from 4% to 18% globally. This translates to increased hospital admissions due to obesity and its related co-morbidities. According to a recent BBC article [2], over a million hospital admissions in 2020 in the UK are attributed to obesity, placing an even greater and unneeded stress on an already stretched National Health Service due to the ongoing COVID-19 pandemic.
Obesity often co-exists with and causes several medical conditions, which all contribute to the patient’s health. Existing medical literature has identified links with diabetes/metabolic syndrome, obstructive sleep apnoea, atherosclerosis leading to increased cardiovascular morbidity, pro-coagulative blood states leading to strokes and deep vein thrombosis, asthma, hypertension and increased risk of cancer.
The UK National Institute for Health and Care Excellence (NICE) guidelines classify obesity according to the patient’s body mass index (BMI) [3]:
-
healthy weight: 18.5 kg/m2 to 24.9 kg/m2
-
overweight: 25 kg/m2 to 29.9 kg/m2
-
obesity I: 30 kg/m2 to 34.9 kg/m2
-
obesity II: 35 kg/m2 to 39.9 kg/m2
-
obesity III: 40 kg/m2 or more.
Discrepancies do exist with merely following the BMI classification—patients with high muscle mass will inadvertently have a higher BMI, although their weight does not consist of much fat. For such cases, waist circumference is accounted for. Current NICE guidance advises clinicians to tackle obesity in three ways:
-
Conservative management: dietary changes, exercise regimes, behavioural interventions and lifestyle interventions.
-
Pharmacological: drugs such as orlistat, reserved for those where conservative management fails.
-
Surgical: using bariatric surgery.
Bariatric surgery
Bariatric surgery is an exciting surgical option that entered the mainstream of weight management in the 1950s, when the first open intestinal bypass surgery was successfully performed. Ever since there have been radical improvements to the various techniques used in bariatric surgery and reduced postoperative complications. In the UK, bariatric surgery is recommended for patients who fulfil specific criteria, including a BMI of more than 40, all appropriate non-surgical interventions have been exhausted, the patient must commit to long term follow up, and more. In the UK, the main types of surgery offered to patients fitting this criteria are gastric banding, gastric bypass, sleeve gastrectomy, intra-gastric balloon, biliopancreatic diversion, and primary obesity surgery-endoluminal [4].
With bariatric surgery becoming a recent but increasingly used procedure for obesity, there is increasing evidence of the various complications arising post-operatively, separate from those directly related to the surgery from a technical perspective. Ophthalmological complications in particular, the focus of this review article, have been increasingly noted with the rising prevalence of this surgery. The limited literature available on this is summarised in this review, such that it helps increase awareness within the community of ophthalmologists and provide a repository of information and evidence for both prospective bariatric surgery patients and their surgeons.
Search strategy
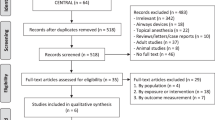
Based on our research question: ‘What are the ophthalmological complications for patients after bariatric surgery?’, we conducted a detailed literature search on the PubMed database. We searched all fields using the key words (‘eye’ OR ‘ophthalmological’ OR ‘ocular’ OR ‘ophthalmic’) AND ‘bariatric surgery’, giving 274 results. Based on the abstracts of these papers, we excluded papers that did not pertain to bariatric surgery as the intervention. We additionally excluded any papers that do not analyse ophthalmic complications related to bariatric surgery, giving us 56 studies that explore ophthalmological links to bariatric surgery.
A search using the keywords above was also conducted using the Cochrane Library, Medline, Embase, Allied and Complementary Medicine and DH-DATA databases. Three additional papers were found, giving us a total of 59 papers for review.
Ophthalmic Associations Of Bariatric Surgery
Nutritional deficiencies
The stomach is a crucial section of the gastrointestinal system that contributes to breakdown, digestion and even absorption of nutrients that are ingested. A study by Kong and Singh [5] explained that this is achieved by storage of food boli, mixing of contents with gastric acid, slow release of the chyme, and release of digestive enzymes (pepsinogen, intrinsic factor, etc). With bariatric surgery, ingested food directly bypasses this crucial conduit or reduces its physical size to suppress appetite and how much food one can physically eat. Such a procedure runs the risk of inducing nutritional deficiencies as a result. A paper delving into nutritional deficiencies caused by bariatric surgery [6] mentions that the main nutrients affected are vitamin A, D, E, K (four fat-soluble vitamins), vitamin B12, B1, C and traces of selenium, iron, zinc, and copper. According to the study, certain bariatric procedures involve restricting food intake (gastric banding, sleeve gastrectomy), while others are malabsorptive (Roux-en-Y gastric bypass [REY], biliopancreatic diversion with duodenal switch). These malabsorptive procedures run the risk of causing ophthalmological pathology related to nutrient loss.
Vitamin A is vital for human life, having a role in reproduction, embryonic growth and development, maintenance of epithelial surfaces, and functioning of the central nervous system [6]. It plays a role in ocular metabolism, including maintenance of the conjunctival and corneal epithelial surfaces and retinal phototransduction and retinal pigment epithelial viability [6]. Many studies support the strong link between vitamin A deficiency (VAD) and xerophthalmia [7]. Commonly known complications of VAD include nyctalopia (night blindness), conjunctival xerosis with Bitot spots, corneal xerosis, corneal ulceration, and keratomalacia [6]. Most of our understanding comes from past epidemics of VAD. Strachan disease, which is characterised by optic, auditory and peripheral neuropathies, and lesions of the skin and mucous membranes, was widely seen in Jamaican sugar cane workers in the 1800s [8]. Similarly in the Cuban epidemic seen in the 1990s, optic neuropathies were rampantly reported [9] amongst the local population, with numbers going beyond 50,000 affected Cubans. The epidemic appeared to be linked to reduced nutrient intake caused by the country’s deteriorating economic situation and the high prevalence of tobacco use [10].
Regarding bariatric surgery and vitamin A levels, most medical literature note an increase in deficiencies. A cohort study published in 2004 found that from a total of 170 patients who had previously undergone biliopancreatic diversion, the incidence of vitamin A deficiency was 69%, vitamin K deficiency 68%, and vitamin D deficiency 63% by the fourth year after surgery [11]. A screening study [12], which got responses from 64 out of 444 patients who underwent REY surgery, found a significant increase in ocular symptoms in this group of patients based on both objective serum vitamin A levels and subjective patient reporting of eyesight. However, as it is a small retrospective screening study, it is prone to various biases. Several case reports also note clinical manifestations of VAD after bariatric surgery. An observational case report by Lee et al. [13] discussed a 39-year-old woman who presented with xerophthalmia and nyctalopia occurring three years after gastric bypass surgery. Another case report discusses a 41-year-old woman who presented with dry eyes, diminished night vision and evident Bitot spots, corneal punctate staining and keratinisation of the conjunctiva [14]. She had a history of bariatric surgery, anaemia, and vitamin D deficiency. The patient was subsequently investigated and found to have a profoundly low serum vitamin A.
On the other hand, a cross-sectional study published in 2017 investigated serum vitamin A in 7 post-vertical sleeve gastrectomy patients and 21 post-REY surgery patients [15]. Other than symptoms of dry eye, the study found that mean vitamin A levels were not different between the two groups, and there was no correlation between serum vitamin A levels and ocular effects. However, this study had a small sample size, and the patients were followed up at an average of 6 months post-surgery, which may not be sufficient time to evaluate ocular changes. The fact remains, however, that VAD is not commonly seen in the developed economies and may not be recognised in its early ophthalmic stages.
Wernicke’s Encephalopathy
While vitamin A seems to be the major factor with eye complications, vitamin B1 (thiamine) deficiencies have been widely noted, manifesting in Wernicke’s encephalopathy (WE) in many patients after undergoing bariatric surgery. Thiamine pyrophosphate (the bioactive form of thiamine) is necessary for energy metabolism in all cells, especially cerebral metabolism. This deficiency can cause metabolic imbalances leading to neurological complications including neuronal cell death. Neuronal death in the mammillary bodies and thalamus were implicated in multiple cases of WE studied [16].
WE is a diagnostic challenge for many clinicians because it is uncommon and presents with a range of subtle features (gait disturbance, altered cognitive state, nystagmus and other eye movement disorders) [17]. WE is commonly associated with alcoholism, which may predispose clinicians to overlook other susceptible patient groups [18]. It is crucial to make an early diagnosis for patients who come with such presenting features and are at high risk. Bariatric patients need to be quickly recognised as such a high-risk group that should be monitored for symptoms associated with WE. Without prompt management, it can cause irreversible damage and progress to Korsakoff’s syndrome.
A literature review exploring the various case reports that diagnosed WE post-bariatric surgery, identified 118 case descriptions in published literature [19]. In many of these cases, vomiting was the most frequently described presenting symptoms and may be a relevant precursor to thiamine deficiency and subsequent WE. Vomiting is noted to be the most common cause of readmission within 30 days for patients undergoing bariatric surgery [20]. The other main causes of readmission were nausea, electrolyte and nutritional depletion. The literature review also found that ataxia was the most common presenting complaint of WE for the bariatric patient group, with 84.7% of cases having this as the primary complaint. Following this is altered mental status, and third being eye movement disorders, such as nystagmus and ophthalmoplegia [20]. Interestingly, the study states that imaging techniques like CT scans and MRI did not give many diagnostic indicators or WE, implying that early presentations need to be clinically diagnosed and immediately managed. Another literature review by Aasheim [21] showed that admission to hospital for WE occurred within 6 months of surgery for 90% of case reports found, which might give clinicians a time frame to hold strong suspicions of WE.
A systematic review by Oudman et al. [22] considered published evidence on a total of 586 non-alcoholic patients who suffered from Wernicke-Korsakoff Encephalopathy. The paper highlights the importance of suspecting WE in non-alcoholic patients, and to look out for signs that may not immediately be diagnostically evident of WE. The study also talks about recommended thiamine doses needed, which might be of interest to clinicians.
Diabetic control and diabetic retinopathy
Diabetic retinopathy (DR) is the most common complication of type 2 diabetes mellitus (T2DM) and is recognised as a microvascular disease. It can be divided in non-proliferative and proliferative DR, where increased vascular permeability and capillary occlusions are noted [23]. This results in ocular oedema, cotton wool spots, microaneurysms, haemorrhages and hard exudates seen on fundoscopy.
Obesity is commonly associated with T2DM among other comorbidities and is a key component to diabetes management. Most patients undergoing bariatric surgery do so to lose weight and hence improve glycaemic control. Many studies [24, 25] have shown better diabetic control (through monitoring of HBA1c levels) after surgical intervention rather than intensive conservative management. However, it is widely debated as to whether rapid weight loss associated with bariatric surgery worsens diabetic retinopathy. There is conflicting evidence for and against this argument, presented in this section.
A non-blinded randomised control trial [26] analysed the difference between bariatric surgery and intensive medical management on diabetic ophthalmic outcomes at 2 years post-surgery. The study involved 150 patients split between intensive medical treatment and surgery (either REY or gastric bypass). While the study found a statistically significant difference in mean change in HBA1c between the medically managed (−1.1) and surgically managed patients (−2.8), it did not find a statistically significant (P = 0.84) worsening or improvement of retinopathy outcomes at 2 years between either group. This was assessed through direct fundoscopy between two ophthalmologists and logMar scales. Another cross-sectional study found from a sample of 96 patients who underwent REY surgery did not show worsening retinopathy in the 6-year follow-up, compared to the sample of 48 non-operated controls who showed worsening retinopathy (using fundus photography for grading) [27]. However, not all the patients involved had fundoscopy grading done prior to bariatric surgery. Another survey study analysed 117 patients who underwent gastric bypass surgery [28]. In this study some patients also had pre-existing DR and were included in the analysis. This study showed that most of the patients showed no deterioration in DR. However, 21 patients out of the 117 showed signs of new or worsening DR. These results are similar to a prospective randomised clinical trial [29], which found that patients who underwent bariatric surgery did not show any changes to visual acuity, but 19.9% of the sample showed some extent of worsening DR. However, the study states that their findings were not statistically significant, and it does not mention the sample size in the abstract. Finally, a systematic review compiling evidence of 12 studies (totalling in 876 patients who underwent bariatric surgery) gave a pooled odds ratio (OR), which showed less DR progression in patients with bariatric surgery than in those with medical treatment alone (OR, 0.47; 95% CI, 0.22–0.99) [30]. However, the review acknowledges a borderline heterogeneity amongst the studies and believes there is still insufficient evidence to assess the effects on DR progression.
On the other hand, there is evidence showing that there is a worsening of DR after bariatric surgery owing to the rapid weight loss and improvement in hyperglycaemia. This may be akin to the so called ‘re-entry normoglycaemic’ response noted in the days when intensive reductions in the blood sugar levels resulted in worsening DR in some patients. This led to changes in clinical practice moderating the intensity and speed with which to reduce hyperglycaemia to guard against worsening DR in the short term. Post bariatric surgery, some studies state that the worsening stage appears from 3 months to 3 years post-surgery [31]. A retrospective observational study of 318 patients with T2DM who underwent bariatric surgery found that 73% had no change to DR grade, 11% regressed and 16% progressed [32]. This range of changes show that although not everyone experiences worsening DR, it is important to screen patients perioperatively to diagnose worsening retinopathy early and intervene immediately for better outcomes. This is a process that is well established for female patients during their pregnancy [33], given that it is known that their DR status can worsen during pregnancy. Another smaller retrospective study (using hospital records) found that out of the 40 patients who had pre- and post-surgery DR screening, 10 patients in total had a certain degree of DR worsening [34]. However, this study had little statistical power due to the small sample size and the retrospective nature with implied biases. One retrospective observational study, however, showed a stark progression in DR [35]. Out of its 102 eligible patients, 24 of them (24%) showed new or progressing retinopathy during the median follow up of 4 years. Finally, a systematic review published in 2021 compiled 14 different studies totalling to 110,300 surgical patients and 252,289 control subjects [36]. The review found that surgical patients had a statistically significantly lower postoperative prevalence of all DR compared to medical management. However, early worsening of DR and progression to sight-threatening DR had occurred more often in those with more severe DR initially. Such findings argue the need for more frequent monitoring of worsening DR during the first postoperative year (see ‘Recommendations’ Section).
Idiopathic intracranial hypertension
Idiopathic Intracranial Hypertension (IIH) is a raised intracranial pressure that is often associated with obesity and the female gender [37]. A study by Hornby et al. [38] explores the possible role of adipose tissue behaving as an endocrine organ, combined with the intestines and brain impacting intracranial pressure and affecting therapeutic options for IIH. This condition usually presents with a combination of one or more of: headaches, vomiting and nausea, neck stiffness, papilloedema and possible abnormal gait. IIH is typically determined by the cerebrospinal spinal fluid (CSF) pressure, however it has been noted that the optic nerve sheath diameter (can be measured using non-invasive ocular ultrasonography) is also an accurate measuring tool for IIH [39]. Increasing obesity rates globally, it has been proposed, are related to increased incidence of IIH. A paper by Mollan et al. [40] discusses the expanding burden of IIH on the healthcare economy. IIH admission and readmission rates are progressively increasing, with admissions rising by 442% between 2002 and 2014. This was seen highest in areas of social deprivation, which is similar to obesity trends across the world.
One of the main management approaches for IIH is weight loss—bariatric surgery is considered for those patients for whom conservative or medical management is insufficient. A randomised controlled trial comparing bariatric surgery versus a community weight loss programme as treatment options for IIH took 66 patients with active IIH and BMI of 35 or more and equally randomised them between the two options [41]. The study found that at 12- and 24-months post-surgery, intracranial pressure (ICP) was significantly lower in the bariatric surgery arm compared to patients who went through conservative weight management. ICP was measured using CSF opening pressure. The study also noted that papilloedema was reduced in both groups after 12 months and quality of life improvement was much greater with bariatric surgery. Visual acuity difference was not significant in either arm. To add to this, another study analysed that bariatric surgery was highly cost-effective compared to conservative management for IIH patients. This is concluded from its lower costs due to reduced readmissions and rapid weight loss, as well as more quality-adjusted life years [42]. These results are backed by a study that reported 16 obese women who underwent sleeve gastrectomy and saw complete resolution in all but two patients [43]. Symptoms reported by this study were chronic headaches, impaired vision, vision loss, papilloedema and field defects. These findings could help ophthalmologists advise patients accordingly about what management option might be preferred.
Apart from bariatric surgery, shunt placement is also an effective management strategy for IIH. The lumboperitoneal shunt moves fluid from the lumbar subarachnoid space to the peritoneum where it is absorbed by the body [44]. A study by Roth et al. [45] compared the outcomes of patients with shunts placed who go through bariatric surgery. The sample of 13 patients did not show any difference between the surgical and non-surgical arms with regards to shunt revisions after. Both groups had a similar number of revisions (0–6 revisions). However, with such a small sample, results were statistically insignificant.
It must be noted that papilloedema may not completely resolve quickly after intervention [46]. A case report explores this - a 37-year-old woman had persistent mild papilloedema 3 months after undergoing bariatric surgery [47].
Retinal microvascular architecture
Retinal structure in obese patients, as reported in the literature, reveals numerous aspects of interest to the ophthalmologist. Severe obesity is associated with neurodegeneration independent of other risk factors like diabetes and metabolic syndrome [48]. It has also been noted that obese patients are at higher risk of developing glaucomatous optic nerve head damage and age-related macular degeneration (AMD) [49]. However, there is still limited evidence exploring the effect of BMI on retinal microvascular architecture. A study by Saban et al. [50] mentions that certain studies claim an increase in choroidal thickness for obese patients (a positive correlation) while others maintain the opposite—reduced choroidal thickness with BMI. The study goes on to state that choroidal thickness is reduced after bariatric surgery with no changes to visual acuity and no new ophthalmological pathology throughout their follow up period of 12 months.
A study by Laiginhas et al. [51], that explores the effect of bariatric surgery on retinal thickness and the optic nerve, reported a process of neurodegeneration in obese patients. This may be correlated to the apparent rise in intraocular pressure (IOP) seen in obesity (see more in the IOP subsection). The study looked at 40 patients, which showed significant thickening of the retina after surgery, independent of diabetic status. This thickening was limited to the inner retinal layers, but there was no reported choroidal thickening, contradictory to the previous study mentioned. The authors also say that there was no evident change to the optic disc and nerve. They hypothesise that this increased retinal thickness implies improved vascularity and growth. The mechanism by which subsequent neurodegeneration develops, as noted by them, is unclear.
Karlsson et al. [52] also performed a comparative trial that showed an increase in arteriole: venule ratio in post-bariatric patients compared to the control arm, suggesting that obesity-related microvascular degeneration is reversible after bariatric surgery-induced weight loss. This is supported by the findings of Brynskov et al. [53], who found that gastric bypass surgery caused a thickening in the retinal nerve fibre layer and the outer nuclear layer of the parafovea, peaking at 6 months post-surgery.
Intraocular pressure
IOP has been shown to be significantly increased in obese patients compared to control groups by a mean of 2.5 mmHg [54]. There are some studies analysing the effect of bariatric surgery and rapid weight loss on IOP and eye outcomes. A comparative observational study looking at 22 obese women versus 15 non-obese women found that IOP was initially higher in the obese group (16.6 ± 3.0 mmHg tonometry), which reduced after bariatric surgery (15.2 ± 2.7 mmHg tonometry) [55]. This drop was further confirmed as reported by Burgansky-Eliash et al. [56], where IOP dropped from 16.9 ± 4 mmHg to 14.1 ± 3 mmHg pre-surgery to post-surgery after 3–6 months. However, the study by Trope et al. [57] acknowledged that although obesity is associated with raised IOP, rapid weight loss is only weakly correlated with reduction in IOP. The implications of this both long term in general and long term for existing ocular hypertensives and glaucoma patients is not known. It will however potentially affect ophthalmic management of these patients.
Obstructive sleep apnoea and the eye
Obstructive Sleep Apnoea (OSA) is strongly associated with obesity, with a study by Young et al. [58] stating that 41% of adult OSA cases are linked to obesity. Obesity is also associated with hypoventilation and the complications that arise from that, known as Pickwickian Syndrome. Interestingly, OSA is also related to various eye disorders among other whole-body physiological impacts. A review paper delves into these complications and states that the literature connects OSA to glaucoma, floppy eyelid syndrome, non-arteritic ischaemic optic neuropathy, keratoconus, AMD and diabetic retinopathy [59]. These are further explored in the next section.
Bariatric surgery has shown improved outcomes regarding OSA. Haines et al. [60] conducted a summative prospective analysis of 349 prospective bariatric patients who went through polysomnography. Out of this group, 101 patients showed reduced respiratory disturbance postoperatively. It was also noted that minimum oxygen saturation, sleep efficiency, and rapid eye movement (REM) latency improved, as well as a reduced need for continuous positive airway pressure. These findings are reinforced by other studies that found that AHI episodes (which are episodes of hypoventilation during the night - normal range is up to 5 episodes per hour) reduced along with a lower percentage of non-REM and higher REM stages [61, 62]. It may be correlated that ophthalmological conditions associated with OSA will improve with bariatric surgery as well, but there is no current evidence showing this.
Miscellaneous
There are less common ocular associations noted post bariatric surgery that can be discussed. A case report by Greenberg and McCormick [63] followed a patient with morbid obesity and bilateral progressive keratoconus. The patient, after undergoing a sleeve gastrectomy, showed a significant improvement in the right keratoconus over 2 years. There was also improved visual acuity along with management of his weight and diabetes. This can be linked to the previous section on OSA and bariatric surgery.
A few cases have also explored the impact of bariatric surgery on tear film and dryness of the eyes. Some studies previously referenced noted dry eyes as a common symptom post-surgery. It has been hypothesised by a study that deficiencies in macro- and micronutrients may cause protein deficiency and subsequently modifications to the lipid tear layer [64]. However, another study refutes this—a randomly selected group of 89 patients post-surgery showed no significant difference in ocular surface disease compared to the control [65]. The study does note a high rate of noncompliance with nutritional supplementation as a confounding factor.
Finally, a case report by Gilchrist et al. [66] found a secondary consequence of low serum vitamin A levels in a patient who underwent biliopancreatic diversion surgery. The patient’s newborn child (7 years after surgery) was noted to have microphthalmia, inferior adherent leukoma and optic nerve hypoplasia. The child had undetectable serum vitamin A levels in the perinatal period.
Recommendations for clinicians
History taking remains the cornerstone of clinical practice. The review of the literature on this topic suggests the potential for closer working between bariatric surgeons and ophthalmologists centred around the patient. While none of the evidence reviewed suggests a bespoke ‘screening service’ or programme, there is enough to warrant consideration of developing clinical practice pathways in ophthalmology aiming to pre-empt ophthalmic complications post bariatric surgery.
Given the evidence discussed above, several options may be available including a pre-operative baseline ophthalmic examination with anterior segment photography, dilated fundus examination and ophthalmic fundus diagnostic imaging. The latter may include widefield imaging, optical coherence tomography (preferably with added angiography) of both the macula and the optic disc. While this array of investigations may be too extensive for bariatric patients, with no evidence that it will be required, it might be worth considering non-mydriatic fundoscopic photographic imaging followed by ophthalmic evaluation and appropriate clinical follow-up for those who show photographic abnormalities [67]. A study by Krispel et al. [68] explored the option of fundoscopic photographic screening in the morbidly obese population and detected 50 patients out of their sample of 606 with fundal abnormalities. Although they concluded that screening would be ineffective in the general morbidly obese population, in the context of bariatric surgery it might be more relevant.
Having ophthalmological imaging for pre-bariatric patients may provide a safety net based on information reviewed in this paper and offer the chance to collect prospective longitudinal real-world data. The opportunity to conduct such a long-term prospective study using predefined criteria entered on a shared register using electronic patient records may well be a matter of time in the making.
Table 1 summarises the potential complications that can arise post-bariatric surgery.
Summary
What was known about this topic
-
Increasing incidence of obesity and rising numbers of patients undergoing bariatric surgery.
-
A variety of ophthalmic pathologies reported post bariatric surgery.
What this paper adds
-
Collates all English language published evidence reporting ocular changes post bariatric surgery.
-
Offers suggestions on different ways of approaching this increasing phenomenon working with bariatric surgery colleagues around the patients’ needs, based on the currently published evidence
References
Obesity [Internet]. WHO international 2021. https://www.who.int/health-topics/obesity#tab=tab_1. Accessed 9 June 2021.
Sophie H. Over a million hospital admissions for obesity [Internet]. BBC News. 2021. https://www.bbc.co.uk/news/health-57144922. Accessed 9 June 2021.
Obesity: identification, assessment and management | Guidance | NICE [Internet]. Nice.org.uk. 2021. https://www.nice.org.uk/guidance/cg189/chapter/1-Recommendations. Accessed 9 June 2021.
Weight loss surgery—Types [Internet]. nhs.uk. 2021. https://www.nhs.uk/conditions/weight-loss-surgery/types/. Accessed 9 June 2021.
Kong F, Singh R. Disintegration of solid foods in human stomach. J Food Sci. 2008;73:R67–R80.
Guerreiro R, Ribeiro R. Ophthalmic complications of bariatric surgery. Obes Surg. 2014;25:167–73.
Vitamin A deficiency and xerophthalmia: report of a Joint WHO/USAID Meeting [held in Jakarta from 25 to 29 November 1974] [Internet]. Apps.who.int. 1974. https://apps.who.int/iris/handle/10665/41197. Accessed 9 June 2021.
Santiesteban-Freixas R, Pamias-González E, Luis-González S, Serrano-Verdecia C, González-Quevedo A, Alfaro-Capdegelle I, et al. Epidemic neuropathy: proposal and arguments to rename Strachan disease as Strachan and Madan disease. Revista de Neurologia [Internet]. 1997; 25. https://europepmc.org/article/med/9528040. Accessed 9 June 2021.
Jefferis J, Hickman S. Treatment and outcomes in nutritional optic neuropathy. Curr Treat Opt Neurol. 2019;21.
Bern C, Philen R, Freedman D, Bowman B, Newman N, Gerr F, et al. Epidemic optic neuropathy in cuba—clinical characterization and risk factors. N Engl J Med. 1995;333:1176–82.
Slater G, Ren C, Siegel N, Williams T, Barr D, Wolfe B, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55.
Eckert M, Perry J, Sohn V, Boden J, Martin M, Rush R, et al. Incidence of low vitamin A levels and ocular symptoms after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:653–7.
Lee W, Hamilton S, Harris J, Schwab I. Ocular complications of hypovitaminosis A after bariatric surgery. Ophthalmology. 2005;112:1031–4.
Crum A, Srikumaran D, Woreta F. Bitot’s spots following bariatric surgery: an ocular manifestation of a systemic disease. Case Rep. Ophthalmol. 2017;8:581–9.
Brandão L, Vilar L, Cavalcanti B, Brandão P, Arantes T, Campos J. Serum levels of vitamin A, visual function and ocular surface after bariatric surgery. Arq Gastroenterol. 2017;54:65–9.
Vasan S, Kumar A. Wernicke Encephalopathy. StatPearls [Internet]. 2020. https://www.ncbi.nlm.nih.gov/books/NBK470344/. Accessed 9 June 2021.
Kohnke S, Meek C. Don’t seek, don’t find: the diagnostic challenge of Wernicke’s encephalopathy. Ann Clin Biochem: Int J Lab Med. 2020;58:38–46.
Scalzo S, Bowden S, Ambrose M, Whelan G, Cook M. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol, Neursurg Psychiatry. 2015;:jnnp-2014-309598.
Oudman E, Wijnia J, van Dam M, Biter L, Postma A. Preventing Wernicke encephalopathy after bariatric surgery. Obes Surg. 2018;28:2060–8.
Berger E, Huffman K, Fraker T, Petrick A, Brethauer S, Hall B, et al. Prevalence and risk factors for bariatric surgery readmissions. Ann Surg. 2018;267:122–31.
Aasheim E. Wernicke Encephalopathy after bariatric surgery. Ann Surg. 2008;248:714–20.
Oudman E, Wijnia J, Oey M, van Dam M, Postma A. Wernicke-Korsakoff syndrome despite no alcohol abuse: a summary of systematic reports. J Neurol Sci. 2021;426:117482.
Wang W, Lo A. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
Kashyap S, Gatmaitan P, Brethauer S, Schauer P. Bariatric surgery for type 2 diabetes: weighing the impact for obese patients. Clevel Clin J Med. 2010;77:468–76.
Keidar A. Bariatric surgery for type 2 diabetes reversal: the risks. Diabetes Care. 2011;34:S361–S266.
Schauer P, Bhatt D, Kirwan J, Wolski K, Aminian A, Brethauer S, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641–51.
Madsen L, Bek T, Richelsen B. Diabetic retinopathy in people with Type 2 diabetes and obesity treated by Roux‐en‐Y gastric bypass compared with non‐operated controls: with focus on the role of diabetes remission in a cross‐sectional and a 6‐year follow‐up study. Diabet. Med. 2018;36:457–64.
Morén Å, Sundbom M, Ottosson J, Granstam E. Gastric bypass surgery does not increase the risk for sight-threatening diabetic retinopathy. Acta Ophthalmol. 2017;96:279–82.
Singh R, Gans R, Kashyap S, Kirwan J, Bedi R, Wolski K, et al. Ophthalmic outcomes of bariatric surgery vs. intensive medical therapy on obese patients with diabetes. Diabetes. 2014;63:A1–A102.
Kim Y, Kim B, Choi B, Sun H, Lee S, Choi K. Bariatric surgery is associated with less progression of diabetic retinopathy: a systematic review and meta-analysis. Surg Obes Relat Dis. 2017;13:352–60.
Bain S, Klufas M, Ho A, Matthews D. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes, Obes Metab. 2018;21:454–66.
Murphy R, Jiang Y, Booth M, Babor R, MacCormick A, Hammodat H, et al. Progression of diabetic retinopathy after bariatric surgery. Diabet Med. 2015;32:1212–20.
Mallika P, Tan A, Aziz S, Asok T, Syed Alwi S, Intan G. Diabetic retinopathy and the effect of pregnancy. Malaysian Family Physician [Internet]. 2010;5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4170393/. Accessed 9 June 2021.
Thomas R, Prior S, Barry J, Luzio S, Eyre N, Caplin S, et al. Does bariatric surgery adversely impact on diabetic retinopathy in persons with morbid obesity and type 2 diabetes? A pilot study. J Diabetes its Complications. 2014;28:191–5.
Chen Y, Laybourne J, Sandinha M, de Alwis N, Avery P, Steel D. Does bariatric surgery prevent progression of diabetic retinopathy? Eye 2017;31:1131–9.
Yu C, Park L, Pinto A, Ma O, Lee Y, Gupta R, et al. The impact of bariatric surgery on diabetic retinopathy: a systematic review and meta-analysis. Am J Ophthalmol. 2021;225:117–27.
Idiopathic Intracranial Hypertension | Cedars-Sinai [Internet]. Cedars-sinai.org. 2021. https://www.cedars-sinai.org/health-library/diseases-and-conditions/p/pseudotumor-cerebri.html#:~:text=Idiopathic%20intracranial%20hypertension%20(IIH)%20is,called%20cerebrospinal%20fluid%20or%20CSF. Accessed 9 June 2021.
Hornby C, Mollan S, Botfield H, O’Reilly M, Sinclair A. Metabolic concepts in idiopathic intracranial hypertension and their potential for therapeutic intervention. J Neuro-Ophthalmol. 2018;38:522–30.
Dip F, Nguyen D, Sasson M, Menzo E, Szomstein S, Rosenthal R. The relationship between intracranial pressure and obesity: an ultrasonographic evaluation of the optic nerve. Surgical Endosc. 2016;30:2321–5.
Mollan S, Aguiar M, Evison F, Frew E, Sinclair A. The expanding burden of idiopathic intracranial hypertension. Eye 2018;33:478–85.
Mollan S, Mitchell J, Ottridge R, Aguiar M, Yiangou A, Alimajstorovic Z, et al. Effectiveness of Bariatric Surgery vs Community Weight Management Intervention for the Treatment of Idiopathic Intracranial Hypertension. JAMA Neurology. 2021;78:678–86.
Elliot L, Frew E, Mollan S, Mitchell J, Yiangou A, Alimajstorovic Z, et al. Cost-effectiveness of bariatric surgery versus community weight management to treat obesity-related idiopathic intracranial hypertension: evidence from a single-payer healthcare system. Surg. Obes. Relat. Dis. 2021;17:1310–6.
Abdelbaki T, Gomaa M. Outcome of idiopathic intracranial hypertension after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2020;16:1195–201.
Idiopathic Intracranial Hypertension Shunting (Pseudotumor Cerebri) [Internet]. Medtronic.com. 2021. https://www.medtronic.com/us-en/healthcare-professionals/therapies-procedures/neurological/shunting-idiopathic-intracranial-hypertension.html#:~:text=In%20the%20case%20of%20IIH,pressure%20needs%20of%20each%20person. Accessed 9 June 2021.
Roth J, Constantini S, Kesler A. Over-drainage and persistent shunt-dependency in patients with idiopathic intracranial hypertension treated with shunts and bariatric surgery. Surgical Neurol Int. 2015;6:655.
Patten J. Neurological differential diagnosis. 2nd ed. London: Springer; 1996.
Simão L, Andrade T, Oliveira e Oliveira A, Garcez A, Jacometti R. Coexistence of papilledema and pseudopapilledema after remission of idiopathic intracranial hypertension by bariatric surgery. Arq. Bras. Oftalmol. 2020;83:157–9.
Laiginhas R, Guimarães M, Cardoso P, Santos-Sousa H, Preto J, Nora M, et al. Retinal nerve fiber layer thickness decrease in obesity as a marker of neurodegeneration. Obes Surg. 2019;29:2174–9.
Posarelli C, Salvetti G, Piaggi P, Guido F, Ceccarini G, Santini F, et al. Ophthalmologic evaluation of severely obese patients undergoing bariatric surgery: a pilot, monocentric, prospective, open-label study. PLOS ONE. 2019;14:e0216351.
Gonul S, Yilmaz H, Gedik S, Ozturk B, Oflaz A, Sahin M. Evaluation of the choroidal thickness and retinal nerve fiber layer and visual fields in morbid obesity: does bariatric surgery affect retinal structure and function? Indian J Ophthalmol. 2021;69:301.
Laiginhas R, Guimarães M, Cardoso P, Santos-Sousa H, Preto J, Nora M, et al. Bariatric surgery induces retinal thickening without affecting the retinal nerve fiber layer independent of diabetic status. Obes Surg. 2020;30:4877–84.
Viljanen A, Soinio M, Cheung C, Hannukainen J, Karlsson H, Wong T, et al. Effects of bariatric surgery on retinal microvascular architecture in obese patients. Int J Obes. 2018;43:1675–80.
Brynskov T, Laugesen C, Floyd A, Sørensen T. Thickening of inner retinal layers in the parafovea after bariatric surgery in patients with type 2 diabetes. Acta Ophthalmol. 2016;94:668–74.
Lam C, Trope G, Buys Y. Effect of head position and weight loss on intraocular pressure in obese subjects. J Glaucoma. 2017;26:107–12.
Viljanen A, Hannukainen J, Soinio M, Karlsson H, Salminen P, Nuutila P, et al. The effect of bariatric surgery on intraocular pressure. Acta Ophthalmol. 2018;96:849–52.
Burgansky-Eliash Z, Achiron A, Hecht I, Shimonov M. Reduction of intraocular pressure after bariatric surgery. Acta Ophthalmol. 2018;96:e592–5.
Trope G, Lam C, Buys Y. Effect of weight loss on intraocular pressure. Investig Ophthalmol Visual Sci [Internet]. 2015;56:95. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01378322/full. Accessed 10 June 2021.
Young T, Peppard P, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9.
West S, Turnbull C. Eye disorders associated with obstructive sleep apnoea. Curr Opin Pulm Med. 2016;22:595–601.
Haines K, Nelson L, Gonzalez R, Torrella T, Martin T, Kandil A, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141:354–8.
Yılmaz Kara B, Kalcan S, Özyurt S, Gümüş A, Özçelik N, Karadoğan D, et al. Weight loss as the first-line therapy in patients with severe obesity and obstructive sleep apnea syndrome: the role of laparoscopic sleeve gastrectomy. Obes Surg. 2020;31:1082–91.
Rao A, Tey B, Ramalingam G, Poh A. Obstructive sleep apnoea (OSA) patterns in bariatric surgical practice and response of OSA to weight loss after laparoscopic adjustable gastric banding (LAGB). Ann Acad Med. Singapore [Internet]. 2009;38. https://pubmed.ncbi.nlm.nih.gov/19652849/. Accessed 10 June 2021.
Greenberg J, McCormick G. Regression of keratoconus after gastric sleeve surgery. Cornea. 2020;39:912–4.
Sánchez-Sánchez A, Rodríguez-Murguía N, Martinez-Cordero C, Chávez-Cerda S. Protein diet in bariatric patients could modify tear film. Obes Surg. 2019;30:2053–5.
Marques N, Felberg S, Barros J, Malheiros C. Evaluation of the ocular surface following bariatric surgery. Arq Bras Oftalmol. 2017;80.
Gilchrist H, Taranath D, Gole G. Ocular malformation in a newborn secondary to maternal hypovitaminosis A. J Am Assoc Pediatr Ophthalmol Strabismus. 2010;14:274–6.
Moss H. Bariatric surgery and the neuro-ophthalmologist. J Neuro-Ophthalmol. 2016;36:78–84.
Krispel C, Keltner J, Smith W, Chu D, Ali M. Undiagnosed papilledema in a morbidly obese patient population. J Neuro-Ophthalmol. 2011;31:310–5.
Author information
Authors and Affiliations
Contributions
Dr Hari helped with structing the first draft and conducting the literature search for relevant evidence to review. He narrowed the number of searches, summarised the findings and contributed to final revisions. Mr Elsherbiny was responsible for recognising the need for a summary paper on ophthalmic complications from bariatric surgery. He shaped the recommendations section and gave input and corrections during the drafting process. He contributed to the final review of the paper prior to publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. No funding received for this review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hari, T., Elsherbiny, S. Bariatric surgery—what the ophthalmologist needs to know. Eye 36, 1147–1153 (2022). https://doi.org/10.1038/s41433-021-01811-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01811-8