Abstract
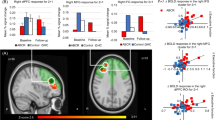
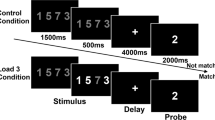
Negative symptoms and cognitive deficits contribute strongly to disability in schizophrenia, and are resistant to existing medications. Recent drug development has targeted enhanced NMDA function by increasing mGluR2/3 signaling. However, the clinical utility of such agents remains uncertain, and markers of brain circuit function are critical for clarifying mechanisms and understanding individual differences in efficacy. We conducted a double-blind, placebo-controlled, randomized cross-over (14 day washout) pilot study evaluating adjunctive use of the mGluR2 positive allosteric modulator AZD8529 (80 mg daily for 3 days), in chronic stable patients with schizophrenia (n = 26 analyzed). We focused on 3 T fMRI response in frontostriatal regions during an n-back working memory task, testing the hypothesis that AZD8529 produces fMRI changes that correlate with improvement in negative symptoms and cognition. We found that AZD8529 did not produce significant group-average effects on symptoms or cognitive accuracy. However, AZD8529 did increase n-back fMRI activation in striatum (p < 0.0001) and anterior cingulate/paracingulate (p = 0.002). Greater drug-versus-placebo effects on caudate activation significantly correlated with greater reductions in PANSS negative symptom scores (r = −0.42, p = 0.031), and exploratory correlations suggested broader effects across multiple symptom domains and regions of interest. These findings demonstrate that fMRI responses to mGluR2 positive modulation relate to individual differences in symptom reduction, and could be pursued for future biomarker development. Negative clinical results at the group level should not lead to premature termination of investigation of this mechanism, which may benefit an important subset of individuals with schizophrenia. Imaging biomarkers may reveal therapeutic mechanisms, and help guide treatment toward specific populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Foussias G, Agid O, Fervaha G, Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur Neuropsychopharmacol. 2014;24:693–709.
Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophrenia Bull. 2015;41:892–9.
Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99:245–53.
Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophrenia Res. 2009;115:74–81.
Anticevic A, Schleifer C, Youngsun TC. Emotional and cognitive dysregulation in schizophrenia and depression: understanding common and distinct behavioral and neural mechanisms. Dialogues Clin Neurosci. 2015;17:421–34.
Foussias G, Siddiqui I, Fervaha G, Mann S, McDonald K, Agid O, et al. Motivated to do well: an examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophrenia Res. 2015;166:276–82.
Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34.
Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–51.
Mucci A, Merlotti E, Ucok A, Aleman A, Galderisi S. Primary and persistent negative symptoms: concepts, assessments and neurobiological bases. Schizophrenia Res. 2017;186:19–28.
Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, et al. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophrenia Bull. 2014;40:1328–37.
Stepien M, Manoliu A, Kubli R, Schneider K, Tobler PN, Seifritz E, et al. Investigating the association of ventral and dorsal striatal dysfunction during reward anticipation with negative symptoms in patients with schizophrenia and healthy individuals. PloS ONE. 2018;13:e0198215.
Wolf DH, Gerraty R, Satterthwaite TD, Loughead J, Campellone T, Elliott MA, et al. Striatal intrinsic reinforcement signals during recognition memory: relationship to response bias and dysregulation in schizophrenia. Front Behav Neurosci. 2011;5:81.
Wolf DH, Turetsky BI, Loughead J, Elliott MA, Pratiwadi R, Gur RE, et al. Auditory oddball fmri in schizophrenia: association of negative symptoms with regional hypoactivation to novel distractors. Brain Imaging Behav. 2008;2:132–45.
Ehrlich S, Yendiki A, Greve DN, Manoach DS, Ho BC, White T, et al. Striatal function in relation to negative symptoms in schizophrenia. Psychological Med. 2012;42:267–82.
Koch K, Wagner G, Nenadic I, Schachtzabel C, Schultz C, Roebel M, et al. Fronto-striatal hypoactivation during correct information retrieval in patients with schizophrenia: an fMRI study. Neuroscience. 2008;153:54–62.
Vink M, Ramsey NF, Raemaekers M, Kahn RS. Striatal dysfunction in schizophrenia and unaffected relatives. Biol Psychiatry. 2006;60:32–9.
Krause M, Zhu Y, Huhn M, Schneider-Thoma J, Bighelli I, Nikolakopoulou A, et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2018;268:625–39.
Omachi Y, Sumiyoshi T. Dose reduction/discontinuation of antipsychotic drugs in psychosis; effect on cognition and functional outcomes. Front Psychiatry. 2018;9:447.
Yang YS, Marder SR, Green MF. Repurposing drugs for cognition in schizophrenia. Clin Pharmacol Ther. 2017;101:191–3.
Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15.
Herman EJ, Bubser M, Conn PJ, Jones CK. Metabotropic glutamate receptors for new treatments in schizophrenia. Handb Exp Pharmacol. 2012;213:297–365.
Ellaithy A, Younkin J, Gonzalez-Maeso J, Logothetis DE. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 2015;38:506–16.
Kawaura K, Karasawa J, Hikichi H. Stimulation of the metabotropic glutamate (mGlu) 2 receptor attenuates the MK-801-induced increase in the immobility time in the forced swimming test in rats. Pharmacol Rep. 2016;68:80–4.
Griebel G, Pichat P, Boulay D, Naimoli V, Potestio L, Featherstone R, et al. The mGluR2 positive allosteric modulator, SAR218645, improves memory and attention deficits in translational models of cognitive symptoms associated with schizophrenia. Sci Rep. 2016;6:35320.
Lavreysen H, Langlois X, Ahnaou A, Drinkenburg W, te Riele P, Biesmans I, et al. Pharmacological characterization of JNJ-40068782, a new potent, selective, and systemically active positive allosteric modulator of the mGlu2 receptor and its radioligand [3H]JNJ-40068782. J Pharmacol Exp Ther. 2013;346:514–27.
Hackler EA, Byun NE, Jones CK, Williams JM, Baheza R, Sengupta S, et al. Selective potentiation of the metabotropic glutamate receptor subtype 2 blocks phencyclidine-induced hyperlocomotion and brain activation. Neuroscience. 2010;168:209–18.
Mehta MA, Schmechtig A, Kotoula V, McColm J, Jackson K, Brittain C, et al. Group II metabotropic glutamate receptor agonist prodrugs LY2979165 and LY2140023 attenuate the functional imaging response to ketamine in healthy subjects. Psychopharmacology. 2018;235:1875–86.
Gray LJ, Hannan AJ, Zhang X. Metabotropic glutamate receptors as targets for novel antipsychotic treatments. Curr Pharm Biotechnol. 2012;13:1522–34.
Acri JB, Cross AJ, Skolnick P. From bench to bedside: mGluR2 positive allosteric modulators as medications to treat substance use disorders. Psychopharmacology. 2017;234:1347–55.
Kent JM, Daly E, Kezic I, Lane R, Lim P, De Smedt H, et al. Efficacy and safety of an adjunctive mGlu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:66–73.
Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–7.
Stauffer VL, Millen BA, Andersen S, Kinon BJ, Lagrandeur L, Lindenmayer JP, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophrenia Res. 2013;150:434–41.
Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, et al. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol. 2011;31:349–55.
Kantrowitz JT, Grinband J, Goff DC, Lahti AC, Marder SR, Kegeles LS, et al. Proof of mechanism and target engagement of glutamatergic drugs for the treatment of schizophrenia: RCTs of pomaglumetad and TS-134 on ketamine-induced psychotic symptoms and pharmacoBOLD in healthy volunteers. Neuropsychopharmacology. 2020;45:1842–50.
Salih H, Anghelescu I, Kezic I, Sinha V, Hoeben E, Van Nueten L, et al. Pharmacokinetic and pharmacodynamic characterisation of JNJ-40411813, a positive allosteric modulator of mGluR2, in two randomised, double-blind phase-I studies. J Psychopharmacol. 2015;29:414–25.
De Boer P, Sinha V, Hoeben E, Ion-George A, Kezic I, Daly E, et al. Characterization of the clinical effect of a positive allosteric modulator of the metabotropic glutamate receptor-2. San Francisco:Poster presented at 68th Annual Scientific Convention of Society of Biological Psychiatry;2013.
Litman RE, Smith MA, Doherty JJ, Cross A, Raines S, Gertsik L, et al. AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: a proof of principle study. Schizophrenia Res. 2016;172:152–7.
Marek GJ. When is a Proof-of-Concept (POC) not a POC? Pomaglumetad (LY2140023) as a case study for antipsychotic efficacy. Curr Pharm Des. 2015;21:3788–96.
Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78:754–62.
Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, et al. mGlur2 versus mGluR3 metabotropic glutamate receptors in primate dorsolateral prefrontal cortex: Postsynaptic mGluR3 strengthen working memory networks. Cereb Cortex. 2018;28:974–87.
Javitt DC, Carter CS, Krystal JH, Kantrowitz JT, Girgis RR, Kegeles LS, et al. Utility of imaging-based biomarkers for glutamate-targeted drug development in psychotic disorders: a randomized clinical trial. JAMA Psychiatry. 2018;75:11–9.
Wandschneider B, Koepp MJ. Pharmaco fMRI: determining the functional anatomy of the effects of medication. Neuroimage Clin. 2016;12:691–7.
Insel TR. The NIMH experimental medicine initiative. World Psychiatry. 2015;14:151–3.
Bhakta SG, Chou HH, Rana B, Talledo JA, Balvaneda B, Gaddis L, et al. Effects of acute memantine administration on MATRICS consensus cognitive battery performance in psychosis: testing an experimental medicine strategy. Psychopharmacology. 2016;233:2399–410.
Sheffler DJ, Gregory KJ, Rook JM, Conn PJ. Allosteric modulation of metabotropic glutamate receptors. Adv Pharmacol. 2011;62:37–77.
Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, et al. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–21.
Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98.
Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82.
Phillips T, Rees S, Augood S, Waldvogel H, Faull R, Svendsen C, et al. Localization of metabotropic glutamate receptor type 2 in the human brain. Neuroscience. 2000;95:1139–56.
Ghose S, Gleason KA, Potts BW, Lewis-Amezcua K, Tamminga CA. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry. 2009;166:812–20.
Johnson KA, Mateo Y, Lovinger DM. Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology. 2017;117:114–23.
Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophrenia Res. 2009;108:143–50.
Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, et al. Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2015;72:456–65.
Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry. 2016;173:517–26.
Rasch B, Papassotiropoulos A, de Quervain DF. Imaging genetics of cognitive functions: focus on episodic memory. NeuroImage. 2010;53:870–7.
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–68.
Backman L, Nyberg L. Dopamine and training-related working-memory improvement. Neurosci Biobehav Rev. 2013;37:2209–19.
Manivannan A, Foran W, Jalbrzikowski M, Murty VP, Haas GL, Tarcijonas G, et al. Association between duration of untreated psychosis and frontostriatal connectivity during maintenance of visuospatial working memory. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:454–61.
Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69.
Li ML, Hu XQ, Li F, Gao WJ. Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: still promising or a dead end? Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:66–76.
Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Lane TA, Boerner T, Bannerman DM, Kew JN, Tunbridge EM, Sharp T, et al. Decreased striatal dopamine in group II metabotropic glutamate receptor (mglu2/mglu3) double knockout mice. BMC Neurosci. 2013;14:102.
Shah UH, Gonzalez-Maeso J. Serotonin and glutamate interactions in preclinical schizophrenia models. ACS Chem Neurosci. 2019;10:3068–77.
Johnson KA, Niswender CM, Conn PJ, Xiang Z. Activation of group II metabotropic glutamate receptors induces long-term depression of excitatory synaptic transmission in the substantia nigra pars reticulata. Neurosci Lett. 2011;504:102–6.
Maltbie EA, Kaundinya GS, Howell LL. Ketamine and pharmacological imaging: use of functional magnetic resonance imaging to evaluate mechanisms of action. Behav Pharm. 2017;28:610–22.
Bryant JE, Frolich M, Tran S, Reid MA, Lahti AC, Kraguljac NV. Ketamine induced changes in regional cerebral blood flow, interregional connectivity patterns, and glutamate metabolism. J Psychiatr Res. 2019;117:108–15.
Bojesen KB, Andersen KA, Rasmussen SN, Baandrup L, Madsen LM, Glenthoj BY, et al. Glutamate levels and resting cerebral blood flow in anterior cingulate cortex are associated at rest and immediately following infusion of S-ketamine in healthy volunteers. Front Psychiatry. 2018;9:22.
Francois J, Grimm O, Schwarz AJ, Schweiger J, Haller L, Risterucci C, et al. Ketamine suppresses the ventral striatal response to reward anticipation: A cross-species translational neuroimaging study. Neuropsychopharmacology. 2016;41:1386–94.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–204.
Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA Jr. Effects of ketamine on brain activity during emotional processing: Differential findings in depressed versus healthy control participants. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:610–8.
Sterpenich V, Vidal S, Hofmeister J, Michalopoulos G, Bancila V, Warrot D, et al. Increased reactivity of the mesolimbic reward system after ketamine injection in patients with treatment-resistant major depressive disorder. Anesthesiology. 2019;130:923–35.
Ahnaou A, de Boer P, Lavreysen H, Huysmans H, Sinha V, Raeymaekers L, et al. Translational neurophysiological markers for activity of the metabotropic glutamate receptor (mGluR2) modulator JNJ-40411813: Sleep EEG correlates in rodents and healthy men. Neuropharmacology. 2016;103:290–305.
Krystal JH, Anticevic A. Toward illness phase-specific pharmacotherapy for schizophrenia. Biol Psychiatry. 2015;78:738–40.
Gill KM, Cook JM, Poe MM, Grace AA. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophrenia Bull. 2014;40:341–50.
Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, et al. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fmri test battery. NeuroImage. 2012;60:1746–58.
Acknowledgements
This study was supported by AstraZeneca Pharmaceuticals LP. DHW was also supported by NIMH grant K23MH085096 and R01MH101111. TDS was supported by NIMH grant R01MH112847, R01MH113550 and ACTTION. The work was also supported by NIMH grants R01MH060722, P50MH064045, and T32MH019112. The authors thank Elizabeth Hanson, Raphael Gerraty, and Janina Seubert for assistance with data acquisition; Jeffrey Valdez for assistance with neuroimaging analysis; Warren Bilker for assistance with statistical analysis; and Monica Calkins for assistance with symptom assessment.
Author information
Authors and Affiliations
Contributions
REG, RCG, BT, SRZ, MAS, AJC, CK, MAE, DHW conceived and designed the study. DW, CK, MEM conducted study procedures and data collection. DW, DZ, KR, TDS, and MEM conducted data analysis. DHW and DZ drafted the paper. All authors reviewed and critically revised the paper and approved of it in its final form.
Corresponding author
Ethics declarations
Competing interests
Drs MAS, SRZ, and AJC are former employees of AstraZeneca Pharmaceuticals LP, the study sponsor. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wolf, D.H., Zheng, D., Kohler, C. et al. Effect of mGluR2 positive allosteric modulation on frontostriatal working memory activation in schizophrenia. Mol Psychiatry 27, 1226–1232 (2022). https://doi.org/10.1038/s41380-021-01320-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01320-w
This article is cited by
-
Elevating the field for applying neuroimaging to individual patients in psychiatry
Translational Psychiatry (2024)
-
Impairments in the early consolidation of spatial memories via group II mGluR agonism in the mammillary bodies
Scientific Reports (2024)
-
Progress and Pitfalls in Developing Agents to Treat Neurocognitive Deficits Associated with Schizophrenia
CNS Drugs (2022)