Abstract
Sodium-glucose cotransporter 2 inhibitors (SGLT2i), initially born as anti-diabetic drugs, have shown many beneficial effects on the cardiovascular system, in particular against heart failure (HF). HF is a complex and multifaceted disease that requires a comprehensive approach. It should not be considered as a simplistic cardiac disease, but a systemic disease that leads to multisystemic organ failure and death. Exploiting their pleiotropic effects, SGLT2i are a very valid tool for HF treatment. Beyond the indication to reduce HF hospitalization and death risk, in patients with diabetes mellitus at high cardiovascular risk or with established cardiovascular event, SGLT2i administration reported beneficial effects regarding the wide spectrum of HF manifestations and stages, independently by diabetes mellitus presence. Recent evidence focuses on HF rehospitalization, cardiac and all-cause death reduction, as well as symptoms and quality of life improvement, in patients with chronic HF or with a recent HF decompensation episode. Given the recent finding about the SGLT2i usefulness in HF patients, further studies are needed to define the best administration timing to maximize the SGLT2i-derived beneficial effects.
Similar content being viewed by others
Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2i), initially born as anti-diabetic drugs, have shown beneficial effects in heart failure (HF) treatment. SGLT2i administration has already been recommended by the European Society of Cardiology (ESC) 2016 Cardiovascular Disease (CVD) Prevention in Clinical Practice Guidelines [1] and then, reconfirmed by the 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases [2] (Table 1). In both cases, SGLT2i use was restricted to diabetic patients with established CVD or at high cardiovascular (CV) risk. The latest observations have shown further benefits deriving from the use of this drugs class, extending their administration regardless of type 2 diabetes mellitus (T2DM) presence and across the wide CV risk factors spectrum. Moreover, many benefits deriving from SGLT2i administration have been observed in HF patients. In fact, currently, Dapagliflozin and Empagliflozin are recommended for the treatment of symptomatic heart failure with reduced ejection fraction (HFrEF), independently by the presence of diabetes mellitus, to reduce HF-related hospitalization and CV death risk [3,4,5,6] (Table 1). SGLT2i are very versatile and suitable in reducing CVD and HF progression. A meta-analysis of the main studies pointed out that SGLT2i, combined with a proper therapy for HF, may significantly reduce HF hospitalization, CV, and all-cause mortality rate, as well as the progression of kidney disease and its related adverse events [7]. Considering the cardio-nephroprotective role, they may slow renal function worsening, providing further benefits on HF-related cardio-renal syndrome. New perspectives regarding the use of SGLT2i are opening, regardless T2DM presence. In this regard, SGLT2i may have protective effects in the HF early phases, when the disease is not clinically overt yet, but morphological or functional or biohumoral heart alterations are already present.
Given the recent findings about the SGLT2i usefulness, also in patients with HF, an important issue concerning the SGLT2i best timing administration is currently ongoing. The aim of this review is to discuss the latest findings regarding SGLT2i use, both in HF and in diabetic patients at risk to develop HF, to provide a better comprehension of the best administration timing, according to patient clinical profile.
The pleiotropic effects of sodium-glucose cotransporter 2 inhibitors: molecular and pathophysiological insights
SGLT2i were approved in 2008 by U.S. Food and Drug Administration (FDA) as anti-diabetic drugs [8]. Type 2 diabetes mellitus (T2DM) and heart are extremely interconnected. T2DM is a major risk factor for HF. Diabetic patients are hospitalized, due to HF, approximately four times more, if compared to non-diabetics [9,10,11,12,13]. In patients with T2DM, the HF development risk is more than twice, compared to non-diabetic population [14]. T2DM not only causes macroangiopathy, but it is itself a diabetic cardiomyopathy’s cause [12, 15]. SGLT2i have shown beneficial effects on CV system, while showing an adequate safety profile. On the contrary, pharmacovigilance studies highlighted that other antidiabetic medications, such as Thiazolidinediones, sulfonylureas, dipeptidyl peptidase 4 (DPP4) inhibitors, and insulin may lead to an increased CV risk [16,17,18,19,20,21,22,23,24].
SGLTs are ubiquitous proteins and, for this reason, SGLT2i show metabolic and hemodynamic effects, which justify their efficacy in T2DM and HF treatment. They slow down harm mechanisms leading to left ventricular remodelling and pathophysiological mechanisms associated with HF development and progression. However, HF is a systemic and progressive disease, and many structural, functional, and biohumoral alterations may appear long time before the overt clinical syndrome [25].
SGLT is a family of sodium glucose cotransporters, and its better-known isoform are SGLT1 and SGLT2. SGLT2 is expressed nearly exclusively in the kidney, while SGLT1 is also present in the intestine and heart [26, 27]. In the kidney, SGLT2 is localized in the first proximal convoluted tubule segment (S1) and exploiting the energy of the Na+/K+ ATPase pump, it transports glucose against gradient, in the peritubular capillaries. It is responsible for the uptake of 90% of the reabsorbed glucose. The other 10% of glucose is then reabsorbed by SGLT1, which is localized in the following proximal convoluted tubules segments (S2 and S3) [28]. Interestingly, SGLT2 is located close to the Na+/H+ exchanger 3 (NHE3), the major responsible for filtered sodium reuptake, in the proximal tubule. The two transporters act together and, for this reason, SGLT2 can directly affect natriuresis [29]. In HF, the NHE3 activity is markedly increased, and it is believed to determine both diuretic and endogenous natriuretic peptides resistance [30, 31]. The myocardium sodium-hydrogen exchanger 1 (NHE 1) hyperactivity results in intracellular sodium and calcium overload [32]. The SGLT2i ability to inhibit NHE-1 entails calcium overload prevention [33]. SGLT2 inhibition causes glycosuria, if glucose blood levels are above 40–80 mg/dL, reducing hypoglycemia risk. The glycosuria-induced osmotic diuretic effect results in volume contraction. Urinary glucose excretion requires, at least, moderately preserved renal function, thus SGLT2i, in particular Dapagliflozin and Empagliflozin, may be administered efficacy and safely up to an estimated glomerular filtration rate (eGFR) of 20–25 ml/min/1.73 m2 [34, 35] (Table 1).
From the pathophysiological point of view, SGLT2i’s effect on arterial blood pressure is determined by preload and afterload reduction. Since diabetic patients have micro- and macrovascular disease, the increased arterial stiffness and afterload promote a vicious circle, through which, the hemodynamic stress, induced by hypertension and reduced arterial blood pressure (BP) variability, causes organ damage. In this context, SGLT2i may play a pleiotropic effect, through which they determine arterial BP reduction [36, 37]. Several studies demonstrated that SGLT2i may improve endothelial function and aortic stiffness indices, inducing vasodilatation through voltage-gated potassium (Kv) channels, and protein kinase G activation [38,39,40,41,42,43,44]. Interestingly, SGLT2i show to selectively reduce interstitial volume with minimal change in vascular volume, whereas loop diuretics may cause a reduction in both interstitial and intravascular volume [45]. In this regard, interstitial space fluid accumulation is responsible for the main HF symptoms, leading to peripheral and pulmonary congestion [46, 47].
Kidney dysfunction is a condition that often affects HF patients [48]. During HF progression, kidney dysfunction is due to HF-related hemodynamic effects, in particular kidney hypoperfusion [49], increased venous congestion and diuretics high doses long-term use [50]. T2DM is frequently associated with nephropathy, characterized by increased intraglomerular pressure and albuminuria. This is due to the afferent renal vessels’ dilation. SGLT2 are able to restore the tubulo-glomerular feedback, bringing more sodium to the macula densa, reducing the afferent vessels vasodilation, the intraglomerular pressure, and the albuminuria of about 40%, slowing down the kidney damage [51]. Dapagliflozin did not cause electrolyte imbalances in a study comparing it to Gliclazide [52]. In particular, the levels of chloride, magnesium, and sulphate were found to be higher, as well as urinary citrate excretion. The latter is a mechanism that contributes to the SGLT2i’s nephroprotective action, as it could be consequent to the citric acid cycle metabolism, the main aerobic energy source for cells [52]. Jhund et al. demonstrated that the Dapagliflozin administration reduces the eGFR reduction rate, showing a similar effect in HFrEF diabetic and non-diabetic patients. Moreover, its efficacy is independent by kidney function at baseline, in terms of HF worsening and CV death risk prevention [53]. Actually, the ongoing Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial is investigating the Dapaglifozin’s efficacy and safety in renal and CV events occurrence reduction, in a population affected by chronic kidney disease, from stage 2 to 4, regardless of T2DM presence [54].
Lowering uric acid level of about 10–15%, SGLT2i contributes to prevent harmful downstream effects, such as inflammation, oxidative stress, and renin angiotensin aldosterone system (RAAS) hyperactivation [55]. This marks another important difference between SGLT2i and other diuretics: the latter are responsible for RAAS hyperactivation, uric acid blood levels increase, electrolytes loss and, above all, metabolic disorders. In fact, SGLT2i do not increase uric blood levels [56] and, although they stimulate tubule-glomerular feedback and induce plasma volume depletion, they determine small variation of serum magnesium, calcium, potassium, and phosphate values and no effects on serum sodium [57]. Moreover, stimulating magnesium blood level increase, SGLT2i have an anti-arrhythmic and cardioprotective effect [58, 59].
Due to urinary glucose loss, SGLT2i promote a direct caloric loss, reducing the body mass index (BMI) and the glycated hemoglobin 1c (HbA1c), between 0.5% and 1.0% [60].
SGLT2i-related urinary volume increase may induce hemoconcentration, hematocrit, as well as serum albumin increase [56, 61]. SGLT2i can restore a better heart oxygenation through hematocrit and hemoglobin levels increase, stopping the vicious cardio-renal involvement, seen in HF [62].
At the metabolic level, SGLT-2i show important implications. In patients with advanced diabetes and HF, fatty acids, and glucose oxidation pathways may be impaired and insufficient. In this context, these drugs induce a metabolic shift in favor of ketone bodies, a much more effective energy source for heart and kidney [63]. In this regard, SGLT2i have been associated with the development of a syndrome-defined euglycemic ketoacidosis. By decreasing the blood glucose values through the glycosuric mechanism, SGLT2i reduce the production of insulin increasing glucagon synthesis, responsible for ketones production, through lipid oxidation [64].
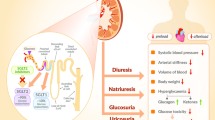
The main SGLT2i molecular and pathophysiological targets and effects are summarized in Fig. 1.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) pleiotropic effects. SGLT2i determine many multisystemic beneficial effects. They reduce volemia, through a natriuretic and glycosuric effect, reducing preload, as well as peripheral and pulmonary congestion. The arterial stiffness reduction promotes vasodilation and arterial pressure reduction. SGLT2i reduce metabolic imbalance and inflammatory response, observed in heart failure and type 2 diabetes mellitus. SGLT2i: sodium-glucose cotransporter2 inhibitors; RAAS: renin–angiotensin–aldosterone system
Type 2 diabetes mellitus and heart failure: role of sodium-glucose cotransporter 2 inhibitors
Many studies and observations underline the effectiveness of SGLT2i use in patients with T2DM, without previous CV events and HF [5]. These observations opened the possibility to use SGLT2i as CV preventive drug, before HF onset, in patients with T2DM. In fact, in these patients, SGLT2i determine adjunctive benefits, reducing both the HF occurrence, as well as HF-related repeated hospitalizations and death. Empagliflozin, Dapagliflozin, Canagliflozin, and Ertugliflozin are actually recommended in patient with T2DM at high CV risk or with stable atherosclerotic cardiovascular disease (ASCVD), to reduce the hospitalization risk, due to HF [5] (Table 1). These observations have encouraged a meta-analysis on three important studies: CANagliflozin cardioVascular Assessment Study (CANVAS) [65], Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) [66] and Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) [67], which confirmed that the Empagliflozin action is equally effective in patients with known HF and in those without [68]. In the CANVAS study, the primary outcome achievement was lower than in the EMPA-REG OUTCOME study and cardiovascular mortality reduction did not reach statistical significance. This could be explained by the different typology of patients enrolled in the two studies [69]. However, both studies show a notable reduction in hospitalization for HF, both in primary and secondary prevention, as long as it is conceivable that the use of SGLT2i in HF may be extended, also in absence of T2DM.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial demonstrated the Canagliflozin’s efficacy in improving renal and CV outcome, in a population composed by patients with T2DM and chronic kidney disease, with or without CV disease. This study opened the possibility to use Canagliflozin to reduce the risk of major CV events and to improve renal function [70].
To reduce the occurrence of HF and other CV events, a strict and early control of CV risk factors is pivotal. In this regard, new evidences underline that SGLT2i act also on other CV risk factors, beyond T2DM, improving patient’s prognosis and reducing the risk of subsequent HF. Guidelines suggest the SGLT2i use in patients with T2DM with at least high CV risk or with ASCVD [1, 2]. In this regard, SGLT2i reduce arterial blood pressure, in patients with T2DM and hypertension, providing benefits in terms of CV risk prevention. It is well known that T2DM and arterial hypertension coexistence markedly worsens the ischemic heart disease, HF and cerebrovascular diseases risk. In fact, up to ¾ of diabetic complications may be due to arterial hypertension [36, 71]. For this reason, arterial blood pressure values control is crucial in diabetic patients, to reduce T2DM-related complications. In those patients, the lack of BP dipping during the night has been observed and it may be due to the increased volemia. Moreover, many diabetic hypertensive patients have masked and nocturnal hypertension, as well as excessive blood pressure rise during the morning, which associate with higher mortality rate [36, 72, 73]. SGLT2i hamper the hemodynamic stress, reducing arterial stiffness, circulating volume overload, and improving endothelial function [36, 74, 75]. Many studies report SGLT2i beneficial effects in the reduction of arterial stiffness and arterial BP profile improvement, shortly after therapy initiation, in diabetic patients [36, 38, 76, 77]. Moreover, Empagliflozin reduced arterial stiffness in younger patients with uncomplicated type 1 diabetes mellitus [36, 78]. SGLT2i contrast obstructive sleep apnea and CV complications, as demonstrated by Sawada et al., who showed apnoea-hypopnea index (AHI) reduction and glycated hemoglobin, as well as BMI improvement, in T2DM patients with obstructive sleep apnoea [36, 79].
As previously defined, in diabetic patients, SGLT2i provide benefits across the wide spectrum of different CV risk factors. This effect occurs both in patients with and without previous CV event. Fitchett et al. studied the Empagliflozin effect, varied by baseline CV risk, on cardiovascular outcome in the EMPA-REG OUTCOME trial [80]. In this context, Empagliflozin reduces HF hospitalization, CV and all-cause mortality, independently by baseline CV risk factors, as well as the presence of prior myocardial infarction and ischemic stroke, in T2DM patients with ASCVD. Moreover, the HF hospitalization reduction was observed both in patients with and without baseline HF. The CV outcomes reduction has been observed in both patients with and without coronary artery bypass grafting (CABG) baseline history [80,81,82] (Table 2). In patients with T2DM and ASCVD, the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV) [83] confirmed that Ertugliflozin is a CV and renal safe and efficient drug.
The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study demonstrates the HF hospitalization risk reduction in patients with T2DM treated with Empagliflozin, regardless of baseline CV disease presence [84] (Table 2). These results are consistent with EMPA-REG OUTCOME trial results, in terms of timing and importance [85]. The Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors (CVD-REAL) study was a real-world study that evaluated the efficacy of SGLT2i in T2DM patients, compared to other glucose-lowering drugs, across several countries [86]. The 87% of patients did not show any known CV disease. The therapeutic management based on SGLT2i determined a reduction in relative risk of 51% for all-cause mortality, 39% for hospitalization due to HF and 46% for the composite of both, compared to other glucose-lowering drug therapeutic plan. Empagliflozin, Dapagliflozin, and Canagliflozin beneficial effect is mainly demonstrated in diabetic patients with high CV risk and regardless of HF, in terms of HF hospitalization prevention. Although SGLT2i effects are notably seen on HF, not all vascular events occurrence may be reduced through SGLT2i administration. In fact, Empagliflozin, Dapagliflozin, Canagliflozin, and Ertugliflozin have a neutral effect regarding stroke and myocardial infarction occurrence prevention [87, 88].
Heart failure and sodium-glucose cotransporter 2 inhibitors: what is the best time of administration?
Despite SGLT2i have been initially used to treat T2DM, increasing evidence emphasize the effectiveness of SGLT2i use in patients with HF, regardless of T2DM presence. Dapagliflozin and Empagliflozin are recommended to reduce HF hospitalization and death in patients with HFrEF [5, 6] and this indication will be probably included in the next ESC HF guidelines (Table 1). HF is a clinical syndrome, as defined by The Universal Definition and Classification of Heart Failure Document [25]. It is characterized by symptoms and/or signs, determined by functional and/or structural cardiac abnormalities, and complemented by systemic or pulmonary congestion and high-circulating natriuretic peptides values [25]. A recently revised HF classification defines 4 stages of disease. Stage A defines patients with risk to develop HF, in which HF risk factors are present without any cardiac or biohumoral alterations. Stage B defines patients with a pre-HF condition, characterized by structural and/or functional cardiac abnormalities or biohumoral alterations, without symptoms. Stage C defines patients with actual or previous HF signs and/or symptoms, determined by a functional and/or structural cardiac alteration. Stage D defines the advanced HF that characterizes patients with severe HF symptoms and/or signs, with frequent recurrences and need of advanced therapies, such as left ventricular assist device, transplant or palliative care, despite an optimized medical therapy [25].
SGLT2i have been used efficiently in different HF stages, from the pre-HF to end-stage HF, as well as in the acute and chronic HF patients. In this context, in HF patients, SGLT2i have demonstrated to significantly reduce rehospitalization, all-cause death, and CV death. Moreover, they improve HF-related symptoms and life quality. This evidence is supported by pathophysiological considerations. In fact, it is simplistic to consider HF as a unique cardiac disease. HF is a multifaceted and complex syndrome characterized by a progressive involvement and dysfunction of systemic organs, such as lung, kidney, liver, brain, and bone marrow. HF could be defined as “the cancer of the heart” because HF management complexity derives from the progressive evolution of systemic dysfunction, leading progressively to multiorgan failure and finally to death. Therefore, as cancer is not treated according to presence of symptoms but to involvement of other organs, according to TNM classification, HF should be treated in order to avoid the progressive involvement and dysfunction of systemic organs, regardless of symptoms presence [89]. Exploiting the cardio-nephroprotective effects, SGLT2 may be administered in HF patients with systemic involvement, mainly characterized by kidney function worsening. A kidney injury, sustained by a low cardiac output, is often observed in HF patients’ clinical scenario, especially in case of HF acute exacerbation. In this case, SGLT2i administration may be considered after an initial patient stabilization, through inodilator and inotropes, if necessary, during the acute phase. However, SGLT2i may demonstrate beneficial effects also in HF patients without an overt systemic involvement, slowing down the systemic disease’s progression and contrasting heart and systemic alterations, that are already present in the early, pre-clinical phases of HF. Considering the SGLT2i pleiotropic and beneficial effects, what type of drug if not SGLT2i may efficiently counteract HF and its multifaceted pathophysiological pathways?
HF natural history is characterized by repeated hospitalizations. Hospitalization and death risks are particularly high during the HF vulnerable phase. This phase identifies the period corresponding to 6 months after an hospitalization due to acute HF [90, 91]. To reduce HF-related mortality and impact on health care system costs, it is important to reduce HF rehospitalization. Savarese et al. showed that the earlier Empagliflozin administration, after an index HF hospitalization, is associated with HF-related rehospitalization and the composite event of all-cause or CV death and HF rehospitalization reduction [90]. Early SGLT2i administration beneficial effect on HF-related adverse events reduction have been identified by the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) trial [92]. This trial demonstrated that the beneficial effect of Empagliflozin on HF hospitalization reduction, urgent and/or emergent HF visit, and death risk became significant starting from the twelfth day from the therapy begin and it is persistent during the treatment period [92] (Table 2). The Empagliflozin impact on HF hospitalization reduction has been demonstrated across several disease’s manifestation severity, inducing up to the 35% of intensive care need reduction. Moreover, the Empagliflozin effect has been observed also in stable and not decompensated HF patients. In this regard, those patients have higher possibility to experience a New York Heart Association (NYHA) class improvement, as well as reduced possibility to experience NYHA class worsening, compared to placebo. Patients treated with Empagliflozin do need less diuretic therapy intensification. Moreover, the SGLT2i effect has been observed also in patients already treated with angiotensin receptor neprilysin inhibitors (ARNI), mineralocorticoid receptor antagonists (MRA), and β-blockers. According to the Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial, the early Sotagliflozin administration, in patients with T2DM and recent HF worsening, was associated with a significant HF hospitalization, urgent visit and CV death reduction, compared to placebo [93] (Table 2). The early Sotagliflozin administration, shortly after, or before a discharge, after an episode of decompensated HF, represents an important opportunity to improve HF outcomes, in diabetic patients. Moreover, the Sotagliflozin beneficial effect have been demonstrated both in subgroups stratified according to left ventricular ejection fraction (LVEF) and the first Sotagliflozin dose administration timing.
In the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial, the primary endpoint was a composite of HF exacerbation, defined as hospitalization for HF and/or urgent visit for intravenous therapy administration, and CV death. The population was made up of HFrEF patients, both with and without diabetes. Dapagliflozin was effective in reducing all individual endpoints and its effectiveness was irrespective of T2DM presence. Dapagliflozin administration improve symptoms and health status in patients with HF, as defined by the Kansas City Cardiomyopathy Questionnaire (KCCQ) [94] (Table 2).
Other aspects in the relationship between HF and SGLT2i have been investigated. In particular, their effectiveness according with disease duration, as well as the effect in HF outpatients and the combined effectiveness of SGLT2i with other drugs administered in HF patients. In this regard, Yeoh et al. analyzed the effect of Dapagliflozin in HF patients of DAPA-HF trial, according to HF duration [95]. In fact, HF is a progressive disease and patients with long-standing HF are older, with more comorbidities and more prone to develop the advanced disease with related complications. Dapagliflozin reduces in similar way the absolute risk of both long standing and recently diagnosed HFrEF patients. However, being the former at higher absolute risk, they reduce more the absolute risk in long standing HF patients, compared with recently diagnosed HFrEF patients. For this reason, Dapagliflozin represents an added value in a great population, the long-standing HF patients, who, having an optimized therapy, are considered not more susceptible of further clinical improvement, through medical treatment. Disease worsening represents a common and important event from a prognostic point of view, in HF outpatients. Docherty et al. conducted a prespecified analysis of DAPA-HF trial in HF outpatients [96]. They demonstrated a reduction of worsening risk in HF outpatients treated with Dapagliflozin, compared to placebo. Moreover, HF worsening, requiring oral therapy management, has the same prognostic meaning, compared to HF outpatients treated intravenously. In fact, disease worsening in HF outpatients leads to oral therapy assumption increase, which is associated with a threefold higher death risk. In these patients, both the HF worsening, requiring therapy management, and hospitalization events, are significantly reduced by Dapagliflozin administration.
Many pathophysiological pathways are involved in HF, and they determine the complexity of this syndrome. For this reason, an early multidrug regimen approach, based on β-blockers, MRA, Levosimendan, ARNI, and SGLT2i, may be beneficial, for HF treatment [97, 98]. Taking data from three important trials, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF), Prospective Comparison of ARNI with ACEI (Angiotensin-Converting–Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF) and DAPA-HF, Vaduganathan et al. evaluated the benefits of a comprehensive therapy, compared to conventional therapy, in chronic HF patients, in terms of overall and free adverse events survival [97]. They found that a comprehensive and early multidrug approach with MRA, beta-blocker, ARNI and SGLT2i gave further benefits, compared to conventional HF therapy (Table 1). In particular, reduction in hospital admission due to HF and CV death has been observed. Patients with a multidrug treatment for HF have demonstrated from 2.7 to 8.3 additional years, without HF hospitalization and CV events, and from 1.4 to 6.3 additional years of survival. Since a multidrug regimen approach for HF treatment can modify the course of HFrEF, the SGLT2i administration is successful and safe and it should be integrated early in HFrEF treatment, regardless of drugs administration order.
Finally, phase III of EMPEROR-preserved trial [99] showed that Empagliflozin significantly reduce the composite outcome of CV death or hospitalization due to HF, in patients with HF with preserved ejection fraction (HFpEF) (Table 1).
Conclusions
HF is a complex disease that requires a comprehensive approach. Due to its multifaceted pathophysiology, HF should not be considered as a simplistic cardiac disease, but a systemic disease that progressively leads to multisystemic organ failure and death [89, 100,101,102,103]. The Universal Definition and Classification of Heart Failure Document includes in the HF definition also patients with risk factors alone and/or initial cardiac and biohumoral alterations, without an overt clinical syndrome [25]. Early identification and treatment of those patients represents a main target for cardiologists, in order to improve patients’ prognosis.
The usefulness of SGLT2i regarding the wide spectrum of HF manifestations and stages have been demonstrated, independently by T2DM presence. Different SGLT2i trials are listed according to the diabetic status and HF presence of patients included (Fig. 2). Two main important points may be extrapolated by recent evidence about the SGLT2i administration. First, SGLT2i beneficial effects have been observed at different HF stages, both in acute and chronic HF patients. In particular, when SGLT2i were administered in patients with recent acute HF and decompensation episode, they demonstrated to significantly modify the disease’s progression, reducing HF rehospitalization, CV, and all-cause death. Secondly, SGLT2i significantly impact the prognosis of patients with T2DM and across the wide CV risk factors spectrum, in terms of HF prevention.
SGLT2i trials, diabetic status, and HF presence. The main trials regarding SGLT2i administration are summarized according to the HF presence and stage, as well as diabetic status of patients included. SGLT2: sodium-glucose cotransporter 2 inhibitors; HF: heart failure; T2DM: type 2 diabetes mellitus
In conclusion, starting from the concept that, similarly to a cancer, HF should be treated in order to avoid the progressive involvement and dysfunction of systemic organs, regardless of symptoms presence, we are proposing that SGLT2i administration should be started as soon as possible, when clinical conditions allow it, regardless of HF hospitalization, mostly for their cardio- and nephroprotective effects. Moreover, their potential application may be already evaluated when morphological, functional or biohumoral heart abnormalities are already present, also in absence of overt HF clinical syndrome, thus not symptom-driven. Although actual SGLT2i indication regards patients with symptomatic chronic HFrEF, SGLT2i may represent a further treatment option to contrast left ventricular remodeling and the progressive systemic involvement seen in HF, in addition to first line therapy.
These observations are challenging the actual guidelines indication about the SGLT2i use, providing new perspectives for the HF patient management.
References
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S, ESC Scientific Document Group (2016) 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 37(29):2315–2381. https://doi.org/10.1093/eurheartj/ehw106
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC, ESC Scientific Document Group (2020) 2019 ESC Guidelines on diabetes pre-diabetes and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41(2):255–323. https://doi.org/10.1093/eurheartj/ehz486
Committee for Medicinal Products for Human Use (CHMP) (2021) Jardiance summary of opinion (post authorisation). https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-jardiance-ii-55_en.pdf. Accessed Jun 2021
Committee for Medicinal Products for Human Use (CHMP) (2020) Summary of opinion (post authorisation). https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-forxiga-ws-1737_en.pdf. Accessed Oct 2020
Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferović J, Jhund PS, Dattilo G, Čelutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainščak M, Jankowska E, Mueller C, Cosentino F, Lund LH, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC (2020) Heart Failure Association of the European Society of Cardiology update on sodium-glucose co-transporter 2 inhibitors in heart failure. Eur J Heart Fail 22(11):1984–1986. https://doi.org/10.1002/ejhf.2026
Committee W, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR (2021) 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol 77(6):772–810. https://doi.org/10.1016/j.jacc.2020.11.022
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396(10254):819–829. https://doi.org/10.1016/S0140-6736(20)31824-9
U.S. Food and Drug Administration (2008) Guidance for industry: diabetes mellitus evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627. Accessed 24 Nov 2017
Mozafarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al (2016) Heart disease and stroke statistics-2016 update: a report from the american heart association. Circulation 133:e38–e360. https://doi.org/10.1161/CIR.0000000000000350
Fitchett DH, Udell JA, Inzucchi SE (2017) Heart failure outcomes in clinical trials of glucose-lowering agents in patients with diabetes. Eur J Heart Fail 19:43–53. https://doi.org/10.1002/ejhf.633
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB et al (2002) Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 106:3068–3072. https://doi.org/10.1161/01.CIR.0000039105.49749.6F
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfeld MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259. https://doi.org/10.1056/NEJMoa052256
Zelniker TA, Braunwald E (2018) Cardiac and renal efects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol 72(15):1845–1855. https://doi.org/10.1016/j.jacc.2018.06.040
Kenny HC, Abel ED (2019) Heart Failure in Type 2 Diabetes Mellitus. Circ Res 124(1):121–141. https://doi.org/10.1161/CIRCRESAHA.118.311371
Seferović PM, Paulus WJ (2015) Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36(27):1718–1727. https://doi.org/10.1093/eurheartj/ehv134
Brands MW, Manhiani MM (2012) Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol 303(11):R1101–R1109. https://doi.org/10.1152/ajpregu.00390.2012
Kaul S, Bolger AF, Herrington D, Giugliano RP, Eckel RH, Association AH, Foundation ACOC (2010) Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American Heart Association and American College Of Cardiology Foundation. J Am Coll Cardiol 55(17):1885–1894. https://doi.org/10.1016/j.jacc.2010.02.014
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre randomised open-label trial. Lancet 373:2125–2135. https://doi.org/10.1016/S0140-6736(09)60953-3
Lincof AM, Wolski K, Nicholls SJ, Nissen SE (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188. https://doi.org/10.1001/jama.298.10.1180
Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559. https://doi.org/10.1056/NEJMoa0802743
Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ et al (2012) Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 157:601–610. https://doi.org/10.7326/0003-4819-157-9-201211060-00003
Roumie CL, Min JY, D’Agostino McGowan L, Presley C, Grijalva CG, Hackstadt AJ et al (2017) Comparative safety of sulfonylurea and metformin monotherapy on the risk of heart failure: a cohort study. J Am Heart Assoc 6(4):e005379. https://doi.org/10.1161/JAHA.116.005379
Packer M (2018) Worsening heart failure during the use of DPP-4 inhibitors: pathophysiological mechanisms clinical risks and potential influence of concomitant antidiabetic medications. JACC Heart Fail 6:445–451. https://doi.org/10.1016/j.jchf.2017.12.016
Page RL, O’Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, Spencer AP, Trupp RJ, Lindenfeld J, American Heart Association Clinical Pharmacology and Heart Failure and Transplantation Committees of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, Council on Quality of Care and Outcomes Research (2016) Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation 134:e32–e69. https://doi.org/10.1161/CIR.0000000000000426
Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez-Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S (2021) Universal definition and classification of heart failure: a report of the Heart Failure Society of America Heart Failure Association of the European Society of Cardiology Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 1:S1071–9164(21)00050–6. https://doi.org/10.1016/j.cardfail.2021.01.022
Wright EM, Turk E (2004) The sodium/glucose cotransport family SLC5. Pflugers Arch 447:510–518. https://doi.org/10.1007/s00424-003-1063-6
Kanai Y, Lee WS, You G, Brown D, Hediger MA (1994) The human kidney low affinity Naþ/glucose cotransporter SGLT2: Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93:397–404. https://doi.org/10.1172/JCI116972
Ghezzi C, Hirayama BA, Gorraitz E, Loo DD, Liang Y, Wright EM (2014) SGLT2 inhibitors act from the extracellular surface of the cell membrane. Physiol Rep 6:e12058. https://doi.org/10.14814/phy2.12058
Packer M (2017) Activation and inhibition of sodiumhydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 136:1548–1559. https://doi.org/10.1161/CIRCULATIONAHA.117.030418
Inoue BH, dos Santos L, Pessoa TD et al (2012) Increased NHE3 abundance and transport activity in renal proximal tubule of rats with heart failure. Am J Physiol Regul Integr Comp Physiol 302(1):R166–R174. https://doi.org/10.1152/ajpregu.00127.2011
Lütken SC, Kim SW, Jonassen T et al (2009) Changes of renal AQP2 ENaC and NHE3 in experimentally induced heart failure. Am J Physiol Renal Physiol 297(6):F1678–F1688. https://doi.org/10.1152/ajprenal.00010.2009
Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G, Fedak PW, Verma S, Dyck JR (2017) Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci 2:347–354. https://doi.org/10.1016/j.jacbts.2017.07.003
Mudaliar S, Alloju S, Henry RR (2016) Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? a unifying hypothesis. Diabetes Care 39:1115–1122. https://doi.org/10.2337/dc16-0542
Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JG, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJ (2019) Clinical practice update on heart failure 2019: pharmacotherapy procedures devices and patient management An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 21(10):1169–1186. https://doi.org/10.1002/ejhf.1531
Rosano GMC, Moura B, Metra M, Böhm M, Bauersachs J, Ben Gal T, Adamopoulos S, Abdelhamid M, Bistola V, Čelutkienė J, Chioncel O, Farmakis D, Ferrari R, Filippatos G, Hill L, Jankowska EA, Jaarsma T, Jhund P, Lainscak M, Lopatin Y, Lund LH, Milicic D, Mullens W, Pinto F, Ponikowski P, Savarese G, Thum T, Volterrani M, Anker SD, Seferovic PM, Coats AJS (2021) Patient profiling in heart failure for tailoring medical therapy A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 23(6):872–881. https://doi.org/10.1002/ejhf.2206
Kario K, Ferdinand KC, O’Keefe JH (2020) Control of 24-hour blood pressure with SGLT2 inhibitors to prevent cardiovascular disease. Prog Cardiovasc Dis 63(3):249–262. https://doi.org/10.1016/j.pcad.2020.04.003
Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, Arcambal A, Kiuchi MG, Head GA, Schlaich MP, Matthews VB (2020) SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci 5(2):169–179. https://doi.org/10.1016/j.jacbts.2019.11.007
Chilton R, Tikkanen I, Cannon CP et al (2015) Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 17:1180–1193. https://doi.org/10.1111/dom.12572
Li H, Shin SE, Seo MS et al (2018) The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci 197:46–55. https://doi.org/10.1016/j.lfs.2018.01.032
Solini A, Giannini L, Seghieri M et al (2017) Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol 16:138. https://doi.org/10.1186/s12933-017-0621-8
Severino P, D’Amato A, Pucci M, Infusino F, Birtolo LI, Mariani MV, Lavalle C, Maestrini V, Mancone M, Fedele F (2020) Ischemic heart disease and heart failure: role of coronary ion channels. Int J Mol Sci 21(9):3167. https://doi.org/10.3390/ijms21093167
Fedele F, Severino P, Bruno N, Stio R, Caira C, D’Ambrosi A, Brasolin B, Ohanyan V, Mancone M (2013) Role of ion channels in coronary microcirculation: a review of the literature. Future Cardiol 9(6):897–905. https://doi.org/10.2217/fca.13.65
Severino P, D’Amato A, Netti L, Pucci M, De Marchis M, Palmirotta R, Volterrani M, Mancone M, Fedele F (2018) Diabetes mellitus and ischemic heart disease: the role of ion channels. Int J Mol Sci 19(3):802. https://doi.org/10.3390/ijms19030802
Severino P, D’Amato A, Netti L, Pucci M, Infusino F, Maestrini V, Mancone M, Fedele F (2019) Myocardial ischemia and diabetes mellitus: role of oxidative stress in the connection between cardiac metabolism and coronary blood flow. J Diabetes Res 2019:9489826. https://doi.org/10.1155/2019/9489826
Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61(10):2108–2117. https://doi.org/10.1007/s00125-018-4670-7
Miller WL (2016) Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail 9(8):e002922. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002922
Assaad S, Kratzert WB, Shelley B, Friedman MB, Perrino A Jr (2018) Assessment of pulmonary edema: principles and practice. J Cardiothorac Vasc Anesth 32(2):901–914. https://doi.org/10.1053/j.jvca.2017.08.028
Liu M, Li XC, Lu L, Cao Y, Sun RR, Chen S, Zhang PY (2014) Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci 18(19):2918–2926
Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, Collins SP, Doehner W, Filippatos GS, Flammer AJ, Fuhrmann V, Lainscak M, Lassus J, Legrand M, Masip J, Mueller C, Papp Z, Parissis J, Platz E, Rudiger A, Ruschitzka F, Schäfer A, Seferovic PM, Skouri H, Yilmaz MB, Mebazaa A (2017) Organ dysfunction injury and failure in acute heart failure: from pathophysiology to diagnosis and management A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 19(7):821–836. https://doi.org/10.1002/ejhf.872
Bartoli E, Rossi L, Sola D, Castello L, Sainaghi PP, Smirne C (2017) Use misuse and abuse of diuretics. Eur J Intern Med 39:9–17. https://doi.org/10.1016/j.ejim.2017.01.016
Piperidou A, Loutradis C, Sarafidis P (2021) SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens 35(1):12–25. https://doi.org/10.1038/s41371-020-00393-4
van Bommel EJM, Geurts F, Muskiet MHA, Post A, Bakker SJL, Danser AHJ, Touw DJ, van Berkel M, Kramer MHH, Nieuwdorp M, Ferrannini E, Joles JA, Hoorn EJ, van Raalte DH (2020) SGLT2 inhibition versus sulfonylurea treatment effects on electrolyte and acid-base balance: secondary analysis of a clinical trial reaching glycemic equipoise: Tubular effects of SGLT2 inhibition in Type 2 diabetes. Clin Sci (Lond) 134(23):3107–3118. https://doi.org/10.1042/CS20201274
Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O’Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJV (2021) Efficacy of Dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 143(4):298–309. https://doi.org/10.1161/CIRCULATIONAHA.120.050391
Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC, Investigators DAPA-CKD (2020) Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35(2):274–282. https://doi.org/10.1093/ndt/gfz290
Lytvyn Y, Perkins BA, Cherney DZ (2015) Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes 39:239–246. https://doi.org/10.1016/j.jcjd.2014.10.013
Van Raalte DH, Bjornstad P, Persson F, Powell DR, de Cassia CR, Wang PS, Liu M, Heerspink HJL, Cherney D (2019) The impact of Sotagliflozin on renal function albuminuria blood pressure and hematocrit in adults with type 1 diabetes. Diabetes Care 42(10):1921–1929. https://doi.org/10.2337/dc19-0937
Cianciolo G, De Pascalis A, Capelli I, Gasperoni L, Di Lullo L, Bellasi A, La Manna G (2019) Mineral and Electrolyte Disorders With SGLT2i Therapy. JBMR Plus 3(11):e10242. https://doi.org/10.1002/jbm4.10242
Ray EC (2020) Evolving understanding of cardiovascular protection by SGLT2 inhibitors: focus on renal protection myocardial effects uric acid and magnesium balance. Curr Opin Pharmacol 54:11–17. https://doi.org/10.1016/j.coph.2020.06.001
Severino P, Netti L, Mariani MV, Maraone A, D’Amato A, Scarpati R, Infusino F, Pucci M, Lavalle C, Maestrini V, Mancone M, Fedele F (2019) Prevention of cardiovascular disease: screening for magnesium deficiency. Cardiol Res Pract 2019:4874921. https://doi.org/10.1155/2019/4874921
Cherney DZI, Cooper ME, Tikkanen I et al (2018) Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure body weight and HbA1c reductions with empagliflozin. Kidney Int 93:231–244. https://doi.org/10.1016/j.kint.2017.06.017
Aberle J, Menzen M, Schmid SM, Terkamp C, Jaeckel E, Rohwedder K, Scheerer MF, Xu J, Tang W, Birkenfeld AL (2020) Dapagliflozin effects on haematocrit red blood cell count and reticulocytes in insulin-treated patients with type 2 diabetes. Sci Rep 10(1):22396. https://doi.org/10.1038/s41598-020-78734-z
Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P (2020) Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 105(4):dgaa057. https://doi.org/10.1210/clinem/dgaa057
Kim JH, Lee M, Kim SH, Kim SR, Lee BW, Kang ES, Cha BS, Cho JW, Lee YH (2019) Sodium-glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter-organ crosstalk. Diabetes Obes Metab 21(4):801–811. https://doi.org/10.1111/dom.13577
Bonora BM, Avogaro A, Fadini GP (2020) Euglycemic Ketoacidosis. Curr Diab Rep 20(7):25. https://doi.org/10.1007/s11892-020-01307-x
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657. https://doi.org/10.1056/NEJMoa1611925
Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, Bluhmki E, Hantel S, Kempthorne-Rawson J, Newman J, Johansen OE, Woerle HJ, Broedl UC (2014) Rationale design and baseline characteristics of a randomized placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOMETM). Cardiovasc Diabetol 13:102. https://doi.org/10.1186/1475-2840-13-102
Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Nicolau JC, Gause-Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD (2019) Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 139(22):2516–2527. https://doi.org/10.1161/CIRCULATIONAHA.119.039996
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Sabatine MS (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393:31–39. https://doi.org/10.1016/S0140-6736(18)32590-X
Scheen AJ (2018) Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res 122(10):1439–1459. https://doi.org/10.1161/CIRCRESAHA.117.311588
Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Capuano G, de Zeeuw D, Greene T, Levin A, Pollock C, Sun T, Wheeler DC, Yavin Y, Zhang H, Zinman B, Rosenthal N, Brenner BM, Perkovic V (2019) Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation 140(9):739–750. https://doi.org/10.1161/CIRCULATIONAHA.119.042007
Epstein M, Sowers JR (1992) Diabetes mellitus and hypertension. Hypertension 19(5):403–418. https://doi.org/10.1161/01.HYP.19.5.403
Sun L, Yan B, Gao Y, Su D, Peng L, Jiao Y, Wang Y, Han D, Wang G (2016) Relationship between blood pressure reverse dipping and type 2 diabetes in hypertensive patients. Sci Rep 6:25053. https://doi.org/10.1038/srep25053
Astrup AS, Nielsen FS, Rossing P, Ali S, Kastrup J, Smidt UM, Parving HH (2007) Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens 5(12):2479–2485. https://doi.org/10.1097/HJH.0b013e3282f06428
Sternlicht H, Bakris GL (2019) Blood pressure lowering and sodium-glucose co-transporter2 inhibitors (SGLT2is): more than osmotic diuresis. Curr Hypertens Rep 21:12. https://doi.org/10.1007/s11906-019-0920-4
Lee DM, Battson ML, Jarrell DK et al (2018) SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol 17:62. https://doi.org/10.1186/s12933-018-0708-x
Ramirez AJ, Sanchez MJ, Sanchez RA (2019) Diabetic patients with essential hypertension treated with amlodipine: blood pressure and arterial stiffness effects of canagliflozin or perindopril. J Hypertens 37:636–642. https://doi.org/10.1097/HJH.0000000000001907
Striepe K, Jumar A, Ott C et al (2017) Effects of the selective sodium-glucose Cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 136:1167. https://doi.org/10.1161/CIRCULATIONAHA.117.029529
Cherney DZ, Perkins BA, Soleymanlou N et al (2014) The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 13:28. https://doi.org/10.1186/1475-2840-13-28
Sawada K, Karashima S, Kometani M et al (2018) Effect of sodium glucose cotransporter 2 inhibitors on obstructive sleep apnea in patients with type 2 diabetes. Endocr J 65:461–467. https://doi.org/10.1507/endocrj.EJ17-0440
Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B (2019) Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME Trial. Circulation 139(11):1384–1395. https://doi.org/10.1161/CIRCULATIONAHA.118.037778
Verma S, Mazer CD, Fitchett D, Inzucchi SE, Pfarr E, George JT, Zinman B (2018) Empagliflozin reduces cardiovascular events mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia 61:1712–1723. https://doi.org/10.1007/s00125-018-4644-9
Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA-REG OUTCOME® trial investigators (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 37(19):1526–1534. https://doi.org/10.1093/eurheartj/ehv728
Cannon CP, McGuire DK, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Charbonnel B, Shih WJ, Gallo S, Masiukiewicz U, Golm G, Cosentino F, Lauring B, Terra SG, Investigators VERTIS-CV (2018) Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J 206:11–23. https://doi.org/10.1016/j.ahj.2018.08.016
Patorno E, Pawar A, Franklin JM, Najafzadeh M, Déruaz-Luyet A, Brodovicz KG, Sambevski S, Bessette LG, Santiago Ortiz AJ, Kulldorff M, Schneeweiss S (2019) Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation 139(25):2822–2830. https://doi.org/10.1161/CIRCULATIONAHA.118.039177
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators EMPA-REGOUTCOME (2015) Empagliflozin cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P, CVD-REAL Investigators and Study Group* (2017) Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation 136(3):249–259. https://doi.org/10.1161/CIRCULATIONAHA.117.029190
Seferović PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, Jhund PS, Polovina MM, Komajda M, Seferović J, Sari I, Cosentino F, Ambrosio G, Metra M, Piepoli M, Chioncel O, Lund LH, Thum T, De Boer RA, Mullens W, Lopatin Y, Volterrani M, Hill L, Bauersachs J, Lyon A, Petrie MC, Anker S, Rosano GMC (2020) European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur J Heart Fail 22(2):196–213. https://doi.org/10.1002/ejhf.1673
Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferović J, Jhund PS, Dattilo G, Čelutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, de Boer RA, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainščak M, Jankowska E, Mueller C, Cosentino F, Lund L, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC (2020) Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 22(9):1495–1503. https://doi.org/10.1002/ejhf.1954
Fedele F, Severino P, Calcagno S, Mancone M (2014) Heart failure: TNM-like classification. J Am Coll Cardiol 63(19):1959–1960. https://doi.org/10.1016/j.jacc.2014.02.552
Savarese G, Sattar N, Januzzi J, Verma S, Lund LH, Fitchett D, Zeller C, George JT, Brueckmann M, Ofstad AP, Inzucchi SE, Wanner C, Zinman B, Butler J (2019) Empagliflozin is associated with a lower risk of post-acute heart failure rehospitalization and mortality. Circulation 139(11):1458–1460. https://doi.org/10.1161/CIRCULATIONAHA.118.038339
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2018) Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137(12):e67–e492. https://doi.org/10.1161/CIR.0000000000000558
Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F (2021) Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: The EMPEROR-reduced trial. Circulation 143(4):326–336. https://doi.org/10.1161/CIRCULATIONAHA.120.051783
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B, SOLOIST-WHF Trial Investigators (2021) Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384(2):117–128. https://doi.org/10.1056/NEJMoa2030183
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA-HF Trial Committees Investigators (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303
Yeoh SE, Dewan P, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Sjöstrand M, Langkilde AM, McMurray JJV, DAPA-HF Investigators Committees (2020) Patient characteristics clinical outcomes and effect of dapagliflozin in relation to duration of heart failure: Is it ever too late to start a new therapy? Circ Heart Fail 13(12)
Docherty KF, Jhund PS, Anand I, Bengtsson O, Böhm M, de Boer RA, DeMets DL, Desai AS, Drozdz J, Howlett J, Inzucchi SE, Johanson P, Katova T, Køber L, Kosiborod MN, Langkilde AM, Lindholm D, Martinez FA, Merkely B, Nicolau JC, O’Meara E, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Tereshchenko S, Verma S, McMurray JJV (2020) Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA-HF. Circulation 142(17):1623–1632. https://doi.org/10.1161/CIRCULATIONAHA.120.047480
Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD (2020) Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 396(10244):121–128. https://doi.org/10.1016/S0140-6736(20)30748-0
Nieminen MS, Buerke M, Parissis J, Ben-Gal T, Pollesello P, Kivikko M, Karavidas A, Severino P, Comín-Colet J, Wikström G, Fedele F (2015) Pharmaco-economics of levosimendan in cardiology: a European perspective. Int J Cardiol 199:337–341. https://doi.org/10.1016/j.ijcard.2015.07.049
Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, EMPEROR-Preserved Trial Committees and Investigators (2019) Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail 21(10):1279–1287. https://doi.org/10.1002/ejhf.1596
Severino P, Maestrini V, Mariani MV, Birtolo LI, Scarpati R, Mancone M, Fedele F (2020) Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail Rev 25(1):9–17. https://doi.org/10.1007/s10741-019-09828-8
Severino P, Mariani MV, Fedele F (2019) Futility in cardiology: the need for a change in perspectives. Eur J Heart Fail 21(11):1483–1484. https://doi.org/10.1002/ejhf.1576
Fedele F, Mancone M, Adamo F, Severino P (2017) Heart failure with preserved mid-range and reduced ejection fraction: the misleading definition of the new guidelines. Cardiol Rev 25(1):4–5. https://doi.org/10.1097/CRD.0000000000000131
Severino P, Mather PJ, Pucci M, D’Amato A, Mariani MV, Infusino F, Birtolo LI, Maestrini V, Mancone M, Fedele F (2019) Advanced heart failure and end-stage heart failure: does a difference exist. Diagnostics (Basel) 9(4):170. https://doi.org/10.3390/diagnostics9040170
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All the Authors have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paolo Severino and Andrea D’Amato contributed equally.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Severino, P., D’Amato, A., Prosperi, S. et al. Sodium-glucose cotransporter 2 inhibitors and heart failure: the best timing for the right patient. Heart Fail Rev 28, 709–721 (2023). https://doi.org/10.1007/s10741-021-10170-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10170-1