Abstract
Purpose
Histamine H3 receptor antagonists and inverse agonists have been extensively developed to treat sleep–wake, neurocognitive, and allied disorders. However, potential adverse effects, including insomnia, hampered the clinical use of these drugs, possibly due to their persistent interaction with the target molecules. The purpose of the present study was to estimate the pharmacokinetics and pharmacodynamics of enerisant, a novel antagonist and inverse agonist for histamine H3 receptors.
Methods
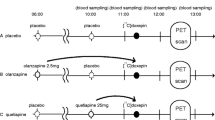
To measure the histamine H3 receptor occupancy by enerisant, positron emission tomography studies using [11C]TASP457, a specific radioligand for histamine H3 receptors, were performed in 12 healthy men at baseline and at 2 h after oral administration of enerisant hydrochloride. For three of these subjects, two additional scans were performed at 6 and 26 h after the administration. Relationships between the receptor occupancy by enerisant and its dose and plasma concentrations were then analyzed.
Results
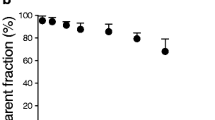
Administration of enerisant hydrochloride decreased the radioligand binding in a dose-dependent manner. The estimated receptor occupancy values at 2 h varied as a function of its dose or plasma concentration. The time course of the occupancy showed persistently high levels (> 85%) in the two subjects with higher doses (25 and 12.5 mg). The occupancy was also initially high at 2 h and 6 h with the lower dose of 5 mg, but it decreased to 69.7% at 26 h.
Conclusion
The target engagement of enerisant was demonstrated in the brains of living human subjects. The occupancy of histamine H3 receptors by enerisant at 2 h can be predicted by applying the plasma concentration of enerisant to Hill’s plot. The preliminary time-course investigation showed persistently high brain occupancy with high doses of enerisant despite the decreasing plasma concentration of the drug. Five milligrams or less dose would be appropriate for the treatment for narcolepsy with initially high occupancy allowing for effective treatment of narcolepsy, and then the occupancy level would be expected to decrease to a level to avoid this drug’s unwanted side effect of insomnia at night, although further research is warranted to confirm the statement since the expected decrease is based on the finding in one subject.
Trial registration
This study was retrospectively registered with ClinicalTrials.gov (NCT04631276) on November 17, 2020.




Similar content being viewed by others
Availability of data and material
The datasets generated and analyzed on the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Brit J Pharmacol. 2008;154:1166–81.
Ghamari N, Zarei O, Arias-Montaño J-A, Reiner D, Dastmalchi S, Stark H, et al. Histamine H3 receptor antagonists/inverse agonists: where do they go? Pharmacol Therapeut. 2019;200:69–84.
Dauvilliers Y, Arnulf I, Szakacs Z, Leu-Semenescu S, Lecomte I, Scart-Gres C, et al. Long-term use of pitolisant to treat patients with narcolepsy: harmony III study. Sleep. 2019;42.
Takano A, Varrone A, Gulyás B, Salvadori P, Gee A, Windhorst A, et al. Guidelines to PET measurements of the target occupancy in the brain for drug development. Eur J Nucl Med Mol. 2016;I(43):2255–62.
Jucaite A, Takano A, Boström E, Jostell K-G, Stenkrona P, Halldin C, et al. AZD5213: a novel histamine H3 receptor antagonist permitting high daytime and low nocturnal H3 receptor occupancy, a PET study in human subjects. Int J Neuropsychoph. 2012;16:1231–9.
Rusjan P, Sabioni P, Ciano PD, Mansouri E, Boileau I, Laveillé A, et al. Exploring occupancy of the histamine H3 receptor by pitolisant in humans using PET. Brit J Pharmacol. 2020;177:3464–72.
Campbell DB. The use of kinetic-dynamic interactions in the evaluation of drugs. Psychopharmacology. 1990;100:433–50.
Hino N, Marumo T, Kotani M, Shimazaki T, Kaku-Fukumoto A, Hikichi H, et al. A novel potent and selective histamine H3 receptor antagonist enerisant: in vitro profiles, in vivo receptor occupancy, wake-promoting and pro-cognitive effects in rodents. J Pharmacol Exp Ther. 2020;375:JPET-AR-2020–000185.
Koga K, Maeda J, Tokunaga M, Hanyu M, Kawamura K, Ohmichi M, et al. Development of TASP0410457 (TASP457), a novel dihydroquinolinone derivative as a PET radioligand for central histamine H3 receptors. Ejnmmi Res. 2016;6:11.
Kimura Y, Seki C, Ikoma Y, Ichise M, Kawamura K, Takahata K, et al. [11C]TASP457, a novel PET ligand for histamine H3 receptors in human brain. Eur J Nucl Med Mol I. 2016;43:1653–63.
Hanyu M, Kawamura K, Takei M, Furutsuka K, Shiomi S, Fujishiro T, et al. Radiosynthesis and quality control of [11C]TASP457 as a clinically useful PET ligand for imaging of histamine H3 receptors in human brain. Nucl Med Biol. 2016;43:679–84.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metabolism. 2007;27:1533–9.
Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metabolism. 2002;22:1271–81.
Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metabolism. 2010;30:46–50.
Av HILL. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (Lond). 1910;40:4–7.
Holford NHG, Sheiner LB. Understanding the dose-effect relationship. Clin Pharmacokinet. 1981;6:429–53.
Akaike H. A new look at the statistical model identification. IEEE T Automat Contr. 1974;19:716–23.
Laere KJV, Sanabria-Bohórquez SM, Mozley DP, Burns DH, Hamill TG, Hecken AV, et al. 11C-MK-8278 PET as a tool for pharmacodynamic brain occupancy of histamine 3 receptor inverse agonists. J Nucl Med. 2014;55:65–72.
Acknowledgements
We thank Takahiro Shiraishi, Takamasa Maeda, and other radiology technologists of the PET Department and members of the Clinical Neuroimaging Team for their support with PET scans; Reiko Sugaya, Kazuko Suzuki, and Shizuko Kawakami for clinical coordination; Hiromi Sano for MRI scans; the staff of the Molecular Probe Program for radioligand syntheses and metabolite analyses; Atsuo Waki and his team for quality assurance of the radioligand; Shinji Mitsuhashi for investigational product administration; and Akiko Nezato and her team for inpatient care. We also thank Ippei Ikusima, M.D., and the staff of SOUSEIKAI Sumida Hospital for subject recruitment, clinical assessments, and follow-ups.
Funding
This work was supported by Taisho Pharmaceutical Co., Ltd.. The precursor and standard of [11C]TASP457 for this study were provided by Taisho Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
YK, TSh, MH, IN, and TSu contributed to the conception and design of this research. YK, KT, SK, CS, and YI participated in data acquisition and preparation. KK and MRZ were in charge of radioligand syntheses and metabolite analyses. YK, TSh, MI, MH, IN, and TSu undertook the analysis and interpretation of imaging data. YK and MI wrote the draft manuscript and managed the literature searches. All authors have made substantial intellectual contribution to the work and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by SOUSEIKAI Hakata Clinic Institutional Review Board and the Radiation Drug Safety Committee and the Institutional Review Board of the National Institute of Radiological Sciences, Japan, and was conducted in compliance with the ethical principles set forth in the Declaration of Helsinki, the standards stipulated in Article 14, Paragraph 3, and Article 80–2 of the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics, and Good Clinical Practice (MHW Ordinance No. 28 dated March 27, 1997).
Consent to participate
All subjects gave written informed consent prior to their inclusion in the study.
Consent for publication
Written informed consent was obtained from all participants regarding publishing their data.
Conflict of interest
Taisho Pharmaceutical Co., Ltd., MH, and TSu hold a patent for [11C]TASP457 and related chemicals as histamine H3 ligands (Japan patent JP2014047209A). TSh and IN are employees of Taisho Pharmaceutical Co., Ltd.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Radiopharmacy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kimura, Y., Takahata, K., Shimazaki, T. et al. Pharmacokinetic and pharmacodynamic assessment of histamine H3 receptor occupancy by enerisant: a human PET study with a novel H3 binding ligand, [11C]TASP457. Eur J Nucl Med Mol Imaging 49, 1127–1135 (2022). https://doi.org/10.1007/s00259-021-05571-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05571-1