Abstract
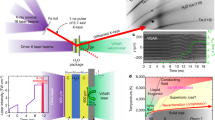
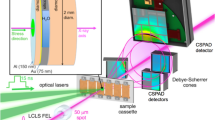
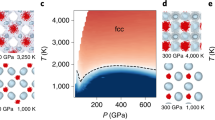
In the phase diagram of water, superionic ices with highly mobile protons within the stable oxygen sublattice have been predicted at high pressures. However, the existence of superionic ices and the location of the melting line have been challenging to determine from both theory and experiments, yielding contradictory results depending on the employed techniques and the interpretation of the data. Here we report high-pressure and high-temperature synchrotron X-ray diffraction and optical spectroscopy measurements of water in a laser-heated diamond anvil cell and reveal first-order phase transitions to ices with body-centred and face-centred cubic oxygen lattices. Based on the distinct density, increased optical conductivity and the greatly decreased fusion enthalpies, we assign these observed structures to the theoretically predicted superionic ice phases. Our measurements determine the pressure–temperature stability fields of superionic ice phases and the melting line, suggesting the presence of face-centred cubic superionic ice in water-rich giant planets, such as Neptune and Uranus. The melting line determined here is at higher temperatures than previously determined in static compression experiments, but it is in agreement with theoretical calculations and data from shock-wave experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Goncharov, A. F., Struzhkin, V. V., Somayazulu, M. S., Hemley, R. J. & Mao, H. K. Compression of ice to 210 gigapascals: infrared evidence for a symmetric hydrogen-bonded phase. Science 273, 218–220 (1996).
Benoit, M., Marx, D. & Parrinello, M. Tunnelling and zero-point motion in high-pressure ice. Nature 392, 258–261 (1998).
Cavazzoni, C. et al. Superionic and metallic states of water and ammonia at giant planet conditions. Science 283, 44–46 (1999).
Benoit, M., Romero, A. H. & Marx, D. Reassigning hydrogen-bond centering in dense ice Phys. Rev. Lett. 89, 145501 (2002).
Goncharov, A. F. et al. Dynamic ionization of water under extreme conditions. Phys. Rev. Lett. 94, 125508 (2005).
Goncharov, A. F. & Crowhurst, J. Proton delocalization under extreme conditions of high pressure and temperature. Phase Transit. 80, 1051–1072 (2007).
Holzapfel, W. B. Symmetry of hydrogen bonds in ice VII. J. Chem. Phys. 56, 712–715 (1972).
Redmer, R., Mattsson, T. R., Nettelmann, N. & French, M. The phase diagram of water and the magnetic fields of Uranus and Neptune. Icarus 211, 798–803 (2011).
Stanley, S. & Bloxham, J. Convective-region geometry as the cause of Uranus’ and Neptune’s unusual magnetic fields. Nature 428, 151–153 (2004).
Schwager, B., Chudinovskikh, L., Gavriliuk, A. & Boehler, R. Melting curve of H2O to 90 GPa measured in a laser-heated diamond cell. J. Phys. Condens. Matter 16, S1177–S1177 (2004).
Lin, J.-F. et al. High pressure-temperature Raman measurements of H2O melting to 22 GPa and 900 K. J. Chem. Phys. 121, 8423–8427 (2004).
Schwager, B. & Boehler, R. H2O: another ice phase and its melting curve. High. Press. Res. 28, 431–433 (2008).
Ahart, M., Karandikar, A., Gramsch, S., Boehler, R. & Hemley, R. J. High P–T Brillouin scattering study of H2O melting to 26 GPa. High. Press. Res. 34, 327–336 (2014).
Frank, M. R., Fei, Y. & Hu, J. Constraining the equation of state of fluid H2O to 80 GPa using the melting curve, bulk modulus, and thermal expansivity of Ice VII. Geochim. Cosmochim. Acta 68, 2781–2790 (2004).
Dubrovinsky, L. & Dubrovinskaia, N. in Advances in High-Pressure Mineralogy (ed. Ohtani, E.) 105–113 (Geological Society of America, 2007).
Datchi, F., Loubeyre, P. & LeToullec, R. Extended and accurate determination of the melting curves of argon, helium, ice (H2O), and hydrogen (H2). Phys. Rev. B 61, 6535–6546 (2000).
Lin, J.-F. et al. Melting behavior of H2O at high pressures and temperatures. Geophys. Res. Lett. 32, L11306 (2005).
Kimura, T., Kuwayama, Y. & Yagi, T. Melting temperatures of H2O up to 72 GPa measured in a diamond anvil cell using CO2 laser heating technique. J. Chem. Phys. 140, 074501 (2014).
Millot, M. et al. Experimental evidence for superionic water ice using shock compression. Nat. Phys. 14, 297–302 (2018).
Méndez, A. S. J. et al. Bulk modulus of H2O across the ice VII–ice X transition measured by time-resolved x-ray diffraction in dynamic diamond anvil cell experiments. Phys. Rev. B 103, 064104 (2021).
Schwegler, E., Sharma, M., Gygi, F. & Galli, G. Melting of ice under pressure. Proc. Natl Acad. Sci. USA 105, 14779–14783 (2008).
Goncharov, A. F. et al. Dissociative melting of ice VII at high pressure. J. Chem. Phys. 130, 124514 (2009).
Sugimura, E. et al. Experimental evidence of superionic conduction in H2O ice. J. Chem. Phys. 137, 194505 (2012).
Aragones, J. L. & Vega, C. Plastic crystal phases of simple water models. J. Chem. Phys. 130, 244504 (2009).
Hernandez, J.-A. & Caracas, R. Superionic-superionic phase transitions in body-centered cubic H2O ice. Phys. Rev. Lett. 117, 135503 (2016).
Hernandez, J.-A. & Caracas, R. Proton dynamics and the phase diagram of dense water ice. J. Chem. Phys. 148, 214501 (2018).
French, M., Mattsson, T. R., Nettelmann, N. & Redmer, R. Equation of state and phase diagram of water at ultrahigh pressures as in planetary interiors. Phys. Rev. B 79, 054107 (2009).
Goldman, N., Fried, L. E., Kuo, I. F. W. & Mundy, C. J. Bonding in the superionic phase of water. Phys. Rev. Lett. 94, 217801 (2005).
Mattsson, T. R. & Desjarlais, M. P. Phase diagram and electrical conductivity of high energy-density water from density functional theory. Phys. Rev. Lett. 97, 017801 (2006).
French, M., Desjarlais, M. P. & Redmer, R. Ab initio calculation of thermodynamic potentials and entropies for superionic water. Phys. Rev. E 93, 022140 (2016).
Wilson, H. F., Wong, M. L. & Militzer, B. Superionic to superionic phase change in water: consequences for the interiors of Uranus and Neptune. Phys. Rev. Lett. 110, 151102 (2013).
Sun, J., Clark, B. K., Torquato, S. & Car, R. The phase diagram of high-pressure superionic ice. Nat. Commun. 6, 8156 (2015).
Millot, M. et al. Nanosecond X-ray diffraction of shock-compressed superionic water ice. Nature 569, 251–255 (2019).
Queyroux, J. A. et al. Melting curve and isostructural solid transition in superionic ice. Phys. Rev. Lett. 125, 195501 (2020).
Ninet, S., Datchi, F. & Saitta, A. M. Proton disorder and superionicity in hot dense ammonia Ice. Phys. Rev. Lett. 108, 165702 (2012).
Rozsa, V., Pan, D., Giberti, F. & Galli, G. Ab initio spectroscopy and ionic conductivity of water under Earth mantle conditions. Proc. Natl Acad. Sci. USA 115, 6952–6957 (2018).
McWilliams, R. S., Dalton, D. A., Mahmood, M. F. & Goncharov, A. F. Optical properties of fluid hydrogen at the transition to a conducting state. Phys. Rev. Lett. 116, 255501 (2016).
Jiang, S. et al. Metallization and molecular dissociation of dense fluid nitrogen. Nat. Commun. 9, 2624 (2018).
Zhang, M., Putnis, A. & Salje, E. K. H. Infrared spectroscopy of superionic conductor LiNaSO4: vibrational modes and thermodynamics. Solid State Ion. 177, 37–43 (2006).
Li, J. et al. Electronic bandgap of water in the superionic and plasma phases. Phys. Plasmas 26, 092703 (2019).
Sun, J. High Pressure Superionic Ice Phase Diagram. PhD thesis, Princeton Univ. (2019).
French, M., Mattsson, T. R. & Redmer, R. Diffusion and electrical conductivity in water at ultrahigh pressures. Phys. Rev. B 82, 174108 (2010).
Mitchell, A. C. & Nellis, W. J. Equation of state and electrical conductivity of water and ammonia shocked to the 100 GPa (1 Mbar) pressure range. J. Chem. Phys. 76, 6273–6281 (1982).
Yakushev, V. V., Postnov, V. I., Fortov, V. E. & Yakysheva, T. I. Electrical conductivity of water during quasi-isentropic compression to 130 GPa. J. Exp. Theor. Phys. 90, 617–622 (2000).
Chau, R., Mitchell, A. C., Minich, R. W. & Nellis, W. J. Electrical conductivity of water compressed dynamically to pressures of 70–180 GPa (0.7–1.8 Mbar). J. Chem. Phys. 114, 1361–1365 (2001).
Lee, K. K. M. et al. Laser-driven shock experiments on precompressed water: Implications for ‘icy’ giant planets. J. Chem. Phys. 125, 014701 (2006).
McWilliams, R. S., Dalton, D. A., Konôpková, Z., Mahmood, M. F. & Goncharov, A. F. Opacity and conductivity measurements in noble gases at conditions of planetary and stellar interiors. Proc. Natl Acad. Sci. USA 112, 7925–7930 (2015).
Duffy, T. S. & Smith, R. F. Ultra-high pressure dynamic compression of geological materials. Front. Earth Sci. 7, 1–20 (2019).
Gómez-Pérez, N. & Heimpel, M. Numerical models of zonal flow dynamos: an application to the ice giants. Geophys. Astrophys. Fluid Dyn. 101, 371–388 (2007).
Helled, R., Anderson, J. D., Podolak, M. & Schubert, G. Interior models of Uranus and Neptune. Astrophys. J. 726, 15 (2010).
Kantor, I. et al. BX90: A new diamond anvil cell design for X-ray diffraction and optical measurements. Rev. Sci. Instrum. 83, 125102 (2012).
Duan, Y. et al. Phase stability and thermal equation of state of δ-AlOOH: Implication for water transportation to the Deep Lower Mantle. Earth Planet. Sci. Lett. 494, 92–98 (2018).
Nisr, C. et al. Large H2O solubility in dense silica and its implications for the interiors of water-rich planets. Proc. Natl Acad. Sci. USA 117, 9747–9754 (2020).
Prakapenka, V. B. et al. Advanced flat top laser heating system for high pressure research at GSECARS: application to the melting behavior of germanium. High. Press. Res. 28, 225–235 (2008).
Shen, G., Rivers, M. L., Wang, Y. & Sutton, S. R. Laser heated diamond cell system at the Advanced Photon Source for in situ X-ray measurements at high pressure and temperature. Rev. Sci. Instrum. 72, 1273–1282 (2001).
Benedetti, L. R. & Loubeyre, P. Temperature gradients, wavelength-dependent emissivity, and accuracy of high and very-high temperatures measured in the laser-heated diamond cell. High. Press. Res. 24, 423–445 (2004).
Prescher, C. & Prakapenka, V. B. DIOPTAS: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High. Press. Res. 35, 223–230 (2015).
Holtgrewe, N., Greenberg, E., Prescher, C., Prakapenka, V. B. & Goncharov, A. F. Advanced integrated optical spectroscopy system for diamond anvil cell studies at GSECARS. High. Press. Res. 39, 457–470 (2019).
Lightfield 5.0 (Teledyne Princeton Instruments, 2016); https://www.princetoninstruments.com/products/software-family/lightfield
Jade 7 (Materials Data, 2008); https://materialsdata.com/prodjd.html
Montoya, J. A. & Goncharov, A. F. Finite element calculations of the time dependent thermal fluxes in the laser-heated diamond anvil cell. J. Appl. Phys. 111, 112617 (2012).
Panero, W. R. & Jeanloz, R. X-ray diffraction patterns from samples in the laser-heated diamond anvil cell. J. Appl. Phys. 91, 2769–2778 (2002).
Deng, J., Du, Z., Benedetti, L. R. & Lee, K. K. M. The influence of wavelength-dependent absorption and temperature gradients on temperature determination in laser-heated diamond-anvil cells. J. Appl. Phys. 121, 025901 (2017).
Lobanov, S. S. & Speziale, S. Radiometric temperature measurements in nongray ferropericlase with pressure- spin- and temperature-dependent optical properties. J. Geophys. Res. Solid Earth 124, 12825–12836 (2019).
Akahama, Y. & Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 310 GPa. J. Appl. Phys. 100, 043516 (2006).
Fei, Y. et al. Toward an internally consistent pressure scale. Proc. Natl Acad. Sci. USA 104, 9182–9186 (2007).
Fischer, R. A. et al. Equations of state and phase boundary for stishovite and CaCl2-type SiO2. Am. Mineral. 103, 792–802 (2018).
Dewaele, A. & Torrent, M. Equation of state of Al2O3. Phys. Rev. B 88, 064107 (2013).
Yen, C. E., Williams, Q. & Kunz, M. Thermal pressure in the laser-heated diamond anvil cell: a quantitative study and implications for the density versus mineralogy correlation of the mantle. J. Geophys. Res. Solid Earth 125, e2020JB020006 (2020).
Anzellini, S. & Boccato, S. A practical review of the laser-heated diamond anvil cell for university laboratories and synchrotron applications. Crystals 10, 459 (2020).
Dewaele, A., Fiquet, G. & Gillet, P. Temperature and pressure distribution in the laser-heated diamond–anvil cell. Rev. Sci. Instrum. 69, 2421–2426 (1998).
Jiang, S. et al. A spectroscopic study of the insulator–metal transition in liquid hydrogen and deuterium. Adv. Sci. 7, 1901668 (2020).
Zha, C.-S., Hemley, R. J., Gramsch, S. A., Mao, H.-K. & Bassett, W. A. Optical study of H2O ice to 120 GPa: Dielectric function, molecular polarizability, and equation of state. J. Chem. Phys. 126, 074506 (2007).
Acknowledgements
Porous carbon samples were received from M. E. Fortunato and K. S. Suslick, University of Illinois at Urbana-Champaign. We thank Z. Geballe for useful comments on the manuscript. This work was performed at GeoSoilEnviroCARS (The University of Chicago, Sector 13), Advanced Photon Source (APS), Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation-Earth Sciences (EAR-1634415) and the Department of Energy-GeoSciences (DE-FG02-94ER14466). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. The work at Carnegie was supported by the NSF (Grant Nos. DMR-1039807, EAR/IF-1128867 and EAR-1763287), the Army Research Office (Grant Nos. 56122-CH-H and W911NF1920172), the Deep Carbon Observatory and the Carnegie Institution of Washington. S.S.L. acknowledges the support of the Helmholtz Young Investigators Group CLEAR (VH-NG-1325).
Author information
Authors and Affiliations
Contributions
V.B.P. and A.F.G. conceived the experiments, V.B.P., N.H., S.S.L. and A.F.G. designed the experiments and V.B.P., N.H. and S.S.L. performed the experiments. V.B.P., N.H. and A.F.G. analysed the data. A.F.G. and V.B.P. wrote the manuscript and all authors reviewed and discussed the manuscript during preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Physics thanks Toshimori Sekine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, Tables 1–4, Figs. 1–18 and refs. 1–29.
Source data
Source Data Fig. 1
Uncompressed ASCII data.
Source Data Fig. 2
Uncompressed ASCII data.
Source Data Fig. 3
Uncompressed ASCII data.
Source Data Fig. 4
Uncompressed ASCII data.
Rights and permissions
About this article
Cite this article
Prakapenka, V.B., Holtgrewe, N., Lobanov, S.S. et al. Structure and properties of two superionic ice phases. Nat. Phys. 17, 1233–1238 (2021). https://doi.org/10.1038/s41567-021-01351-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-021-01351-8
This article is cited by
-
Rich proton dynamics and phase behaviours of nanoconfined ices
Nature Physics (2024)
-
Diamond precipitation dynamics from hydrocarbons at icy planet interior conditions
Nature Astronomy (2024)
-
Double superionicity in icy compounds at planetary interior conditions
Nature Communications (2023)
-
Melting curve of superionic ammonia at planetary interior conditions
Nature Physics (2023)
-
Ammonia and the ice giants
Nature Physics (2023)