Abstract
Aim
To estimate the risk of blindness with primary angle-closure glaucoma (PACG) compared to primary open-angle glaucoma (POAG) in those population-based studies that reported blindness rates for both PACG and POAG.
Method
A systematic search was performed in PubMed for articles published in English between 2000 and 2020 reporting the prevalence of POAG as well as PACG among various ethnic populations. A study was included if it was (1) population-based (2) had published prevalence and blindness rates for both PACG and POAG in the same cohort. (3) Glaucoma was defined as per the International Society for Geographical and Epidemiological Ophthalmology (ISGEO) criteria. The proportion of blindness for both POAG and PACG for each study and the cumulative proportion taking all the studies were calculated.
Results
We included 23 studies with 78,434 participants. POAG was diagnosed in 1702 persons with 151 (8.9%) blind. There were 724 cases of PACG with 196 (27.0%) blind. The risk ratio of blindness in PACG to POAG varied from 0.73 to 10.6 among the studies. The cumulative risk ratio was 2.39 (95% confidence interval (CI); 1.99, 2.87). Risk ratios for studies including visual field restriction while defining blindness were similar to studies that did not (1.92 vs 2.64, P = 0.11). Risk ratios were also similar for studies that used greater than 2 instead of 3 or more quadrants of iridotrabecular contact to define angle closure (2.79 vs 2.25).
Conclusion
Primary angle-closure disease is more likely to be associated with blindness.
Similar content being viewed by others
Introduction
Glaucoma is the leading cause for irreversible blindness across the world. While both primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG) can cause blindness, various population-based studies have reported higher rates of blindness among eyes with PACG compared to POAG [1,2,3]. In PACG, one of the important underlying mechanisms is pupillary block. This can result in acute spikes of very high intraocular pressure (IOP) or more commonly chronic asymptomatic disease where intermittent angle-closure results in IOP spikes, disc damage and progressive synechial closure. This could explain why PACG is the more destructive form of disease than POAG. The prevalence of PACG varies among different racial and ethnic groups with the highest rates reported in Inuit [4] and Asian populations, with studies in African and European populations reporting the lowest rates. It is estimated that PACG accounts for about 40% of all primary glaucoma in Asian populations [5]. However, there are wide variations in the prevalence of disease, rates of blindness and sample sizes used to assess these rates in different populations. This meta-analysis aims to estimate the risk of blindness with PACG compared to POAG in those population-based studies that reported blindness rates for both conditions.
Methods
The ethics committee at Sankara Nethralaya, Chennai approved this study. The review followed the Meta-analysis of Observational Studies in Epidemiology guidelines for the conduct of systematic reviews and meta-analyses of observational studies [6].
Literature search
We performed a systematic search in PubMed for articles published in English between 2000 and 2020 reporting the prevalence of POAG as well as PACG among various ethnic populations. The search was done from 2000 since the prototype system of International Society for Geographical and Epidemiological Ophthalmology (ISGEO) guidelines [7] started being developed by the working group from 1998 and was published in 2002. We used the keywords “population based”, “primary”, “angle”, “closure”, “glaucoma” and “prevalence”. A separate search for articles “population based”, “primary”, “open”, “angle”, “glaucoma” and “prevalence” within the same time period was also performed. All abstracts were reviewed and the population-based studies meeting the inclusion criteria were identified and articles were retrieved. In order to ensure completeness, cross references of the identified articles were searched to identify studies that met the inclusion criteria and were not identified in the PubMed search. The initial literature search was completed by two reviewers (RG and SP) independently. Disagreements between the two were resolved by discussion and a common consensus was obtained.
Inclusion criteria
We included a study if it met the following criteria: (1) Population-based recruitment, in which population-based surveys used a random or cluster sampling procedure, to include participants from a defined geographic region who were representative of the population of that region. (2) Should have published prevalence and blindness rates for both PACG and POAG in the same cohort. (3) Glaucoma should have been defined as per the ISGEO definitions.
Data extraction
The data extracted from each eligible study included: country of study, year of publication, response rate, sample size, the definition of blindness, number of quadrants of angle closure taken into consideration, number of POAG and angle-closure glaucoma cases with number of blind patients in each group. The studies were divided based on the population setting, whether urban/rural or both, the definition of blindness considered in the study and the number of quadrants of closure taken while defining angle-closure disease.
Risk of bias assessment
We used modified Leboeuf-Yde and Lauritsen tool [8] which consists of ten items that address external validity and internal validity of each study and a summary to assess the risk of bias. Studies were reported as low risk (≥7 parameters with low risk of bias), medium (4 to 6 parameters with low risk of bias) and high risk of bias (<4 parameters with low risk of bias). Overall evidence was qualified using GRADE system [9] (Grading of Recommendations Assessment, Development, and Evaluation).
The bias assessment and GRADE evidence profile evaluation were performed by two reviewers (RG and SP) independently. Disagreements between the two were resolved with discussion.
Data analysis
All the statistical analysis was done using Review Manager (RevMan) Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We calculated the proportion of blindness for both POAG and PACG individually for each population-based study as well as the cumulative proportion taking all the population studies. The PACG versus POAG risk ratio with 95% confidence intervals were calculated. Each study was assigned a weight of dependent on the variance of the study. Heterogeneity across all the included studies was tested using the chi-square test using the I2 value [10] which remains unaffected by the number of studies included unlike the chi-square value. A chi-square value of <0.1 was considered statistically significant proving homogeneity in the studies and I2 value of <25% meant a low heterogeneity among the eligible studies. We pooled the data using the random effects model. We also studied the effects of rural versus urban residence, the use of visual field, best-corrected visual acuity (BCVA) taken while defining blindness, and whether the case definition of PACG was based on iridotrabecular contact in 3 or 2 quadrants.
Results
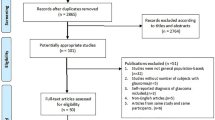
Our PubMed search using both sets of keywords listed 105 articles for PACG and 208 for POAG (Fig. 1). Titles and abstracts of 313 articles were screened, of which 27 were duplicates and 246 were excluded for not meeting any of the inclusion criteria. Full texts of the remaining 40 articles were screened. An additional four articles were identified from the articles referenced in these publications. Thirty articles were selected and reviewed by two of the authors. We finally identified 23 studies (Table 1) that met all the inclusion and exclusion criteria.
All the included studies had low risk of bias (Supplementary Table 1). All the studies included were glaucoma prevalence studies, which reported blindness as a secondary outcome. The decision to publish would not be contingent on the presence/absence of blindness, therefore we considered the chance of publication bias to be negligible. Hence it was not calculated.
Most of the studies were from Asia with six from China, four were from India, four from Singapore, two from Japan and one each from Korea, Myanmar, Nepal and Bangladesh. One study was from Iran and one each from USA and Brazil.
Three studies included both urban and rural populations with 1 study (APEDS) reporting prevalence in both settings separately. Fifteen studies reported prevalence from either rural (10) or urban (5) settings. Five studies did not specify a rural or urban location of the study population. The examination rate in the included studies ranged from 72% to 90.2% with an average of 79.9%. Although the definition of POAG was almost similar in all studies, based on ISGEO criteria, the definition of angle-closure glaucoma varied. Closure or apposition of greater than or equal to two quadrants of the angle was considered angle closure by eight studies, 13 studies defined angle closure as three quadrants or more and 1 study did not specifically define the extent of angle closure (Proyecto VER). One study (Bangladesh) defined two-thirds closure (240°) or more of apposition as angle closure. For purposes of analysis, this study was included in the 270° angle-closure category. The definition of blindness varied among the studies. The cut-off of visual acuity varied with cut-off of 2/40 (Log MAR 1.3 or equivalent) in 17 studies and 2/20(Log MAR 1 or equivalent) in 5 studies. Visual field constriction was included in the definition of blindness by nine studies, and one study (Yazd, Iran) did not specify the definition of blindness. A participant was considered blind if the criteria was satisfied in at least one eye. Out of 78,434 participants of all studies, POAG was diagnosed in 1702, of which 151 (8.9%) were blind. There were 724 cases of PACG with 196 (27.0%) blind. The risk ratio of blindness in PACG to POAG for individual studies varied from 0.73 to 10.6. The cumulative risk ratio was 2.39 (95% confidence interval (CI); 1.99, 2.87) with an overall effect 9.4 (p < 0.00001) (Fig. 2).
There were 41,046 participants from rural and 16,321 from urban areas. In the rural population, 96 out of 793 POAG and 149 of 473 PACG cases were blind. In the urban population 24 of 408 POAG and 24 of 133 PACG cases were blind. A similar higher risk of blindness with PACG was seen in both in rural populations (risk ratio: 2.37 (95% CI; 1.79, 3.13)) and urban populations (risk ratio: 2.65 (95% CI; 1.54, 4.57)) (Fig. 3). Studies that were done in mixed populations also showed a similar difference in risk 3.01 (95% CI; 0.65, 13.94).
We included 31,658 participants (49 blind/556 POAG, 87 blind /307 PACG) from 8 studies that included visual field restriction while defining blindness. Risk ratios for studies that included visual field restriction were similar 1.92(95% CI; 1.39, 2.67) to studies that did not 2.64 (95% CI; 2.12, 3.3) (Fig. 4). A similar higher risk of blindness with PACG was seen in studies defining blindness with BCVA < 6/60 or equivalent (risk ratio: 2.42 (95% CI; 1.56, 3.76)) and <3/60 or equivalent (risk ratio: 2.4 (95% CI; 1.95, 2.97)) (Supplementary Fig. 1). There were 34,803 participants (70 blind/750 POAG, 66 blind/263 PACG) in studies where greater than 2 quadrants of iridotrabecular contact to define angle closure and, 38,857 participants (76 blind/858 POAG, 130 blind/456 PACG) in studies which used 3 quadrants or more to define angle closure. Risk ratios were also similar for studies that used greater than two quadrants 2.79 (95% CI; 1.81, 4.31) versus three quadrants 2.25(95% CI; 1.75, 2.89) (Supplementary Fig. 2).
GRADE assessment
As mentioned above, overall evidence was qualified using GRADE system. We did not consider to start grading as low evidence (as done for an observational study) as these are population-based epidemiological studies and not interventional studies. Overall quality of evidence that PACG is potentially more blinding condition compared to POAG was high (Supplementary Table 2).
Discussion
Primary angle-closure disease was associated with significantly greater risk of blindness in this meta-analysis. This corresponds with what has been suspected in clinical practice. Intermittent spikes or acute elevations in IOP may cause greater optic nerve damage than the more gradual elevation in IOP seen in POAG.
Inclusion of studies following ISGEO guidelines ensured uniformity of the diagnostic definition. Earlier studies had used varying definitions for angle-closure disease. Foster et al. reported a higher prevalence of angle-closure glaucoma in Mongolia [11] compared to other non-Asian studies. They also defined angle closure based on visibility of angle structures seen and POAG suspects, similar to category 2 glaucoma in the ISGEO guidelines. This approach helped fill lacunae in diagnostic criteria and was further refined as the ISGEO guidelines [7].
We compared rural and urban populations for two reasons. Improved access to care in urban areas may result in higher cataract surgical rates which in turn could translate into lower angle-closure disease rates [12]. Second, two studies [1, 13] from India reported a significantly higher prevalence of POAG in an urban setting compared to a rural population and we wanted to assess any impact on blindness rates as a consequence. We did note a statistically higher prevalence of POAG (408/16,321) in the urban populations versus the rural population (793/41,046) (p = 0.0001). An increase in POAG prevalence may not translate into increased severity of disease and would possibly explain the similar pattern in glaucoma blindness.
The inclusion of visual field criteria or the BCVA taken to define blindness did not significantly impact differences in blindness between POAG and PACG. The cumulative prevalence of blindness for PACG in the visual field group was 22.3% and 26.3% in the visual acuity group. For POAG these figures were 11.9% and 10.2%, respectively. Since we had head-to-head comparisons for individual studies that used the same criteria for both diseases this is expected. One could argue that with increased blindness rates in PACG there would be more eyes with advanced visual field damage without loss of foveal acuity and therefore studies that reported blindness using visual field criteria in addition to visual acuity criteria could potentially have higher PACG: POAG blindness ratios. The small numbers of blind persons in individual studies probably accounts for the lack of an association.
Differences in the gonioscopic diagnostic criteria used for Primary angle-closure disease (two quadrants versus three) did not impact the blindness ratio either. This is again intuitive since an eye with PACG with advanced disease causing visual/perimetric blindness is likely to have long-standing disease with more extensive angle damage which would be detected by either criterion. The differences in gonioscopic parameters are likely to impact earlier stages of the disease.
We tried to minimize bias by including only those studies that used standardized disease definitions, appropriate patient sampling, study design and that reported blindness from both POAG and PACG from the same cohort. We excluded studies that had reported blindness from POAG or PACG alone. This was, in some studies, because of very small numbers with either POAG or PACG with no explicit information regarding blindness in the smaller disease cohort. Inclusion of these studies could alter the results with the risk of bias.
Limitations of the study
The data in the current study is mostly from Asian countries and thus is less representative of the whole world. For the same reason, there are fewer studies from Western populations that have reported substantial numbers of people with angle-closure disease and therefore blindness rates could not be compared in these regions.
Conclusion
This meta-analysis reiterates what has been reported in many individual studies that PACG caused significantly (Risk ratio: 2.39; 95% CI: 1.99, 2.87) more blindness than POAG. This is a cause for concern as PACG is prevalent in Asia which is expected to have one of the largest increases in population over the next few decades [14]. This is also a region with poor glaucoma detection rates. A sustained effort to improve diagnostic rates and initiation of appropriate treatment will be required to prevent avoidable angle-closure-related blindness.
Summary
What was known before
-
Glaucoma is a blinding condition: Various population-based studies have reported higher blindness rates in eyes with PACG compared to POAG. The prevalence of PACG, rates of blindness varies among different racial and ethnic groups. The variations: The sample sizes used, population setting, the definition of blindness to assess these rates in different populations are different.
What this study adds:
-
This metanalysis concludes that primary angle-closure glaucoma is a potentially more blinding condition (risk ratio- 2.4) in spite of the variations in the population-based studies.
References
Garudadri C, Senthil S, Khanna RC, Sannapaneni K, Rao HBL. Prevalence and risk factors for primary glaucomas in adult urban and rural populations in the Andhra Pradesh eye disease study. Ophthalmology. 2010;117:1352–9.
Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in North China: the Beijing Eye Study. Am J Ophthalmol. 2010;150:917–24.
Vijaya L, George R, Arvind H, Baskaran M, Ve Ramesh S, Raju P, et al. Prevalence of primary angle-closure disease in an urban south Indian population and comparison with a rural population. The Chennai Glaucoma Study. Ophthalmology. 2008;115:655–60.
Bourne RR, Sørensen KE, Klauber A, Foster PJ, Johnson GJ, Alsbirk PH. Glaucoma in East Greenlandic Inuit—a population survey in Ittoqqortoormiit (Scoresbysund): - a population survey in Ittoqqortoormiit (Scoresbysund). Acta Ophthalmol Scand. 2001;79:462–7.
Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42.
Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–9.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hövsgöl province, northern Mongolia. Arch Ophthalmol. 1996;114:1235–41.
Hu C-C, Lin H-C, Chen C-S. A 7-year population study of primary angle closure glaucoma admissions and climate in Taiwan. Ophthalmic Epidemiol. 2008;15:66–72.
Vijaya L, George R, Baskaran M, Arvind H, Raju P, Ramesh SV, et al. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population. The Chennai Glaucoma Study. Ophthalmology. 2008;115:648–54.
Foster PJ. The prevalence of glaucoma in Chinese residents of Singapore, A cross-sectional population survey of the tanjong pagar district. Arch Ophthalmol. 2000;118:1105.
Thapa SS, Paudyal I, Khanal S, Twyana SN, Paudyal G, Gurung R, et al. A population-based survey of the prevalence and types of glaucoma in Nepal: the Bhaktapur Glaucoma Study. Ophthalmology. 2012;119:759–64.
Vijaya L, George R, Arvind H, Baskaran M, Paul PG, Ramesh SV, et al. Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol. 2006;124:403–9.
Vijaya L, George R, Paul PG, Baskaran M, Arvind H, Raju P, et al. Prevalence of open-angle glaucoma in a rural south Indian population. Investig Ophthalmol Vis Sci. 2005;46:4461–7.
Rahman MM, Rahman N, Foster PJ, Haque Z, Zaman AU, Dineen B, et al. The prevalence of glaucoma in Bangladesh: a population based survey in Dhaka division. Br J Ophthalmol. 2004;88:1493–7.
Liang YB, Friedman DS, Zhou Q, Yang X, Sun LP, Guo LX, et al. Prevalence of primary open angle glaucoma in a rural adult Chinese population: the Handan eye study. Investig Ophthalmol Vis Sci. 2011;52:8250–7.
Liang Y, Friedman DS, Zhou Q, Yang XH, Sun LP, Guo L, et al. Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: the Handan Eye Study. Investig Ophthalmol Vis Sci. 2011;52:8672–9.
Qu W, Li Y, Song W, Zhou X, Kang Y, Yan L, et al. Prevalence and risk factors for angle-closure disease in a rural Northeast China population: a population-based survey in Bin County, Harbin. Acta Ophthalmol. 2011;89:e515–20.
Sun J, Zhou X, Kang Y, Yan L, Sun X, Sui H, et al. Prevalence and risk factors for primary open-angle glaucoma in a rural northeast China population: a population-based survey in Bin County, Harbin. Eye. 2012;26:283–91.
Song W, Shan L, Cheng F, Fan P, Zhang L, Qu W, et al. Prevalence of glaucoma in a rural northern china adult population: a population-based survey in Kailua county, inner Mongolia. Ophthalmology. 2011;118:1982–8.
Sawaguchi S, Sakai H, Iwase A, Yamamoto T, Abe H, Tomita G, et al. Prevalence of primary angle closure and primary angle-closure glaucoma in a southwestern rural population of Japan: the Kumejima Study. Ophthalmology. 2012;119:1134–42.
Yamamoto S, Sawaguchi S, Iwase A, Yamamoto T, Abe H, Tomita G, et al. Primary open-angle glaucoma in a population associated with high prevalence of primary angle-closure glaucoma: the Kumejima Study. Ophthalmology. 2014;121:1558–65.
He M, Foster PJ, Ge J, Huang W, Zheng Y, Friedman DS, et al. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Investig Ophthalmol Vis Sci. 2006;47:2782–8.
Casson RJ, Newland HS, Muecke J, McGovern S, Abraham L, Shein WK, et al. Prevalence of glaucoma in rural Myanmar: the Meiktila Eye Study. Br J Ophthalmol. 2007;91:710–4.
Kim C-S, Seong GJ, Lee N-H, Song K-C, Namil Study Group, Korean Glaucoma Society. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011;118:1024–30.
Kim YY, Lee JH, Ahn MD, Kim CY, Namil Study Group, Korean Glaucoma Society. Angle closure in the Namil study in central South Korea. Arch Ophthalmol. 2012;130:1177–83.
Sakata K, Sakata LM, Sakata VM, Santini C, Hopker LM, Bernardes R, et al. Prevalence of glaucoma in a South brazilian population: Projeto Glaucoma. Investig Ophthalmol Vis Sci. 2007;48:4974–9.
Quigley HA. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819.
Shen SY, Wong TY, Foster PJ, Loo J-L, Rosman M, Loon S-C, et al. The prevalence and types of glaucoma in malay people: the Singapore Malay eye study. Investig Ophthalmol Vis Sci. 2008;49:3846–51.
Narayanaswamy A, Baskaran M, Zheng Y, Lavanya R, Wu R, Wong W-L, et al. The prevalence and types of glaucoma in an urban Indian population: the Singapore Indian Eye Study. Investig Ophthalmol Vis Sci. 2013;54:4621–7.
Baskaran M, Foo RC, Cheng C-Y, Narayanaswamy AK, Zheng Y-F, Wu R, et al. The prevalence and types of glaucoma in an urban Chinese population: the Singapore Chinese eye study: The Singapore Chinese eye study. JAMA Ophthalmol. 2015;133:874–80.
Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–8.
Yamamoto T, Iwase A, Araie M, Suzuki Y, Abe H, Shirato S, et al. The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112:1661–9.
Raychaudhuri A, Lahiri SK, Bandyopadhyay M, Foster PJ, Reeves BC, Johnson GJ. A population based survey of the prevalence and types of glaucoma in rural West Bengal: the West Bengal Glaucoma Study. Br J Ophthalmol. 2005;89:1559–64.
Pakravan M, Yazdani S, Javadi M-A, Amini H, Behroozi Z, Ziaei H, et al. A population-based survey of the prevalence and types of glaucoma in central Iran: the Yazd eye study. Ophthalmology. 2013;120:1977–84.
Zhong H, Li J, Li C, Wei T, Cha X, Cai N, et al. The prevalence of glaucoma in adult rural Chinese populations of the Bai nationality in Dali: the Yunnan Minority Eye Study. Investig Ophthalmol Vis Sci. 2012;53:3221–5.
Author information
Authors and Affiliations
Contributions
RG and LV—Conception and design of this paper. RG and SP—Literature search and data extraction. RG, SP and LV—Statistical analysis and paper draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41433_2021_1802_MOESM2_ESM.tif
Supplementary figure 1: Comparison of risk ratios for blindness between PACG and POAG based on BCVA taken for definition of blindness
41433_2021_1802_MOESM3_ESM.tif
Supplementary figure 2: Comparison of risk ratios for blindness between PACG and POAG based on number of quadrants taken to define angle closure disease.
Rights and permissions
About this article
Cite this article
George, R., Panda, S. & Vijaya, L. Blindness in glaucoma: primary open-angle glaucoma versus primary angle-closure glaucoma—a meta-analysis. Eye 36, 2099–2105 (2022). https://doi.org/10.1038/s41433-021-01802-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01802-9