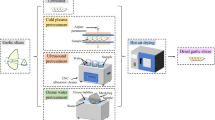
Abstract
Purpose
Analyze the effect of HCl concentration 0.24 mol as a synthesis catalyst on the viscosity of CA-LBG and determine the effect of the application of CA-LBG as a disintegrating agent on the physical quality of tablets.
Methods
Citric acid-locust bean gum (CA-LBG) was synthesized from citric acid (CA) and locust bean gum (LBG) using hydrochloric acid (HCl) and ultraviolet irradiation (UV 254 nm, 100 min). The CA-LBG was analyzed by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), scanning electron microscopy (SEM), esterification efficiency, solubility, and viscosity. The tablet formulation used CA-LBG with a concentration variation of 0.5%, 1%, 2%, 4%, 8%, and 12%. Preparation of tablets by direct compression uses a spray dray lactose (SDL) as a filler with a tablet weight of 200 mg.
Results
Synthesis conditions using 0.24 mol HCl to produce CA-LBG 9.48 cP. The presence of CA-LBG as a disintegrating agent has variation effects to thickness, break force, tensile strength, and friability according to the concentration used. In the formulation process, increasing the concentration of CA-LBG in the tablet mass decreased the flow rate and increased compressibility.
Conclusion
The increase in the concentration of CA-LBG in tablets accelerated the disintegration of tablets without the influence of other tablet parameters. The CA-LBG disintegration activity through repulsion between CA-LBG deformations on the tablet when wetted with disintegration medium. The repulsion force occurs due to the character of CA-LBG which has low solubility and low viscosity.
Graphical Abstract












Similar content being viewed by others
References
Das N, Triparthi N, Basu S, Bose C, Maitra S, Khurana S. Progress in the development of gelling agents for improved culturability of microorganisms. Front Microbiol. 2015;6:1–7.
Dey P, Maiti S, Sa B. Novel etherified locust bean gum-alginate hydrogels for controlled release of glipizide. J Biomater Sci Polym Ed. 2013;24:663–83.
Dionísio M, Grenha A. Locust bean gum: exploring its potential for biopharmaceutical applications. J Pharm Bioallied Sci. 2012;4:175–85.
Sheskey PJ, Cook WG, Cable CG. Handbook of pharmaceutical excipients 8th. London-Washington DC: Pharmaceutical Press and American Pharmacists Association; 2017.
Chudzikowski RJ. Guar gum and its applications. J Soc Cosmet Chem. 1971;22:43–60.
Hadinugroho W, Martodihardjo S, Fudholi A, Riyanto S. Esterification of citric acid with locust bean gum. Heliyon. Elsevier Ltd; 2019; 5:e02337. https://doi.org/10.1016/j.heliyon.2019.e02337.
Hadinugroho W, Martodihardjo S, Fudholi A, Riyanto S. Study of a catalyst of citric acid crosslinking on locust bean gum. J Chem Technol Metall. 2017;52:1086–91.
Samavati V, Razavi SH, Rezaei KA, Aminifar M. Intrinsic viscosity of locust bean gum and sweeteners mixture in dilute solutions. Electron J Environ Agric Food Chem. 2007;6:1879–89.
Tamaki Y, Teruya T, Tako M. The chemical structure of galactomannan isolated from seeds of Delonix regia. Biosci Biotechnol Biochem. 2010;74:1110–2.
Bhattacharya A, Rawlins JW, Ray P. Polymer grafting and crosslinking. Polym. Grafting Crosslink. Canada: A John Wiley & Sons, Inc, Publication; 2008.
Colas A. Cow corning silicones : preparation properties and performance. Materials. Midland; 2005.
Santiago EV, Lopez SHA, Romero AR. Photochemical cross-linking study of polymers containing diacetylene groups in their main chain and azobenzene compounds as pendant groups. Superf y vacío. 2006;19:1–7.
Tjandraatmadja GF, Burn LS, Jollands MJ. The effects of ultraviolet radiation on polycarbonate glazing. Proc 8th Int Conf Durab Build Constr Mater Vancouver, Canada. 1999;30:884–98.
Yeh CC, Chen CN, Li YT, Chang CW, Cheng MY, Chang HI. The effect of polymer molecular weight and UV radiation on physical properties and bioactivities of PCL films. Cell Polym. 2011;30:261–76.
Markl D, Zeitler JA. A review of disintegration mechanisms and measurement techniques. Pharm Res Pharmaceutical Research. 2017;34:890–917.
Gulrez SKH, Al-Assaf S, Phillips GO. Hydrogels: methods of preparation, characterisation and applications. Prog Mol Environ Bioeng - From Anal Model to Technol Appl. 2011.
Aulton ME, Taylor K. Aulton’s pharmaceutics the design and manufacture of medicines. BMC Public Health. New York: Churchill Livingstone Elsevier; 2017.
The United States Pharmacopeial Convention. Pharmacopeia 41-National Formulary 36. 41st ed. Rockville: Twinbrook Parkway; 2018.
Shang C, Sinka IC, Jayaraman B, Pan J. Break force and tensile strength relationships for curved faced tablets subject to diametrical compression. Int J Pharm. Elsevier B.V.; 2013;442:57–64. https://doi.org/10.1016/j.ijpharm.2012.09.005.
Pitt KG, Newton JM, Richardson R, Stanley P. The material tensile strength of Convex-faced aspirin tablets. J Pharm Pharmacol. 1989;41:289–92.
Kumar MU, Babu MK. Design and evaluation of fast dissolving tablets containing diclofenac sodium using fenugreek gum as a natural superdisintegrant. Asian Pac J Trop Biomed. Hainan Medical University; 2014;4:S329–34. https://doi.org/10.12980/APJTB.4.2014B672.
Hammami MM, Hussein RF, Alswayeh R, Alvi SN. Eight enteric-coated 50 mg diclofenac sodium tablet formulations marketed in Saudi Arabia: in vitro quality evaluation. BMC Res Notes BioMed Central. 2020;13:1–6. https://doi.org/10.1186/s13104-020-05270-4.
Bertocchi P, Antoniella E, Valvo L, Alimonti S, Memoli A. Diclofenac sodium multisource prolonged release tablets-a comparative study on the dissolution profiles. J Pharm Biomed Anal. 2005;37:679–85.
Zupančič Bozič D, Vrečer F, Kozjek F. Optimization of diclofenac sodium dissolution from sustained release formulations using an artificial neural network. Eur J Pharm Sci. 1997;5:163–9.
Ghasemi J, Niazi A, Ghobadi S. Simultaneous spectrophotometric determination of benzyl alcohol and diclofenac in pharmaceutical formulations by chemometrics method. J Chinese Chem Soc. 2005;52:1049–54.
Gouda AA, Kotb El-Sayed MI, Amin AS, El Sheikh R. Spectrophotometric and spectrofluorometric methods for the determination of non-steroidal anti-inflammatory drugs: a review. Arab J Chem. 2013;6:145–63. https://doi.org/10.1016/j.arabjc.2010.12.006.
Coates J. Interpretation of infrared spectra, a practical approach. Encycl Anal Chem. 2006;10815–37.
Doll KM, Shogren RL, Willett JL, Swift G. Solvent-free polymerization of citric acid and D-sorbitol. J Polym Sci Part A Polym Chem. 2006;44:4259–67.
Jans AWH, Kinne RKH. 13C NMR spectroscopy as a tool to investigate renal metabolism. Kidney Int. 1991;39:430–7.
Zhang YL, Zhao CX, Liu XD, Li W, Wang JL, Hu ZG. Application of poly(aspartic acid-citric acid) copolymer compound inhibitor as an effective and environmental agent against calcium phosphate in cooling water systems. J Appl Res Technol. 2016;14:425–33.
Azero EG, Andrade CT. Characterisation of Prosopis juliflora seed gum and the effect of its addition to κ-carrageenan systems. J Braz Chem Soc. 2006;17:844–50.
Bhatia H, Gupta PK, Soni PL, Division C. Extraction, purification and characterization of a galactomannan from Prosopis juliflora (Sw.) Dc. Seed. Int J Sci Enviroment Technol. 2013;2:708–24.
Gillet S, Aguedo M, Blecker C, Jacquet N, Richel A. Use of 13C-NMR in structural elucidation of polysaccharides: case of locust bean gum. Young Belgium Magn. Reson. Sci. Liege: University of Liege; 2014.
Parvathy KS, Susheelamma NS, Tharanathan RN, Gaonkar AK. A simple non-aqueous method for carboxymethylation of galactomannans. Carbohydr Polym. 2005;62:137–41.
Szumilo M, Belniak P, Swiader K, Holody E, Poleszak E. Assessment of physical properties of granules with paracetamol and caffeine. Saudi Pharm J King Saud University. 2017;25:900–5. https://doi.org/10.1016/j.jsps.2017.02.009.
Directorate General of Medicine and Food. Indonesian Pharmacopoeia. 4th ed. Jakarta: Ministry of Health Republic of Indonesia; 1995.
Acknowledgements
The authors thank the research, technology, and higher education department, Indonesia, for support of this work by providing research grants (0299/E3/2016). The author also thanks PT. Makmur Food (Indonesia) for supporting locust bean gum (Viscogum); LPPT Gadjah Mada University (Indonesia) for the SEM, DSC, and NMR instrument support; Faculty of Pharmacy, Gadjah Mada University (Indonesia), for support of pharmaceutical technology facilities; and Faculty of Pharmacy, Widya Mandala Catholic University Surabaya (Indonesia), for pharmaceutical technology facilities and instruments.
Author information
Authors and Affiliations
Contributions
Wuryanto Hadinugroho conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools, or data; and wrote the paper.
Suwaldi Martodihardjo, Achmad Fudholi, and Sugeng Riyanto conceived and designed the experiments and analyzed and interpreted the data.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hadinugroho, W., Martodihardjo, S., Fudholi, A. et al. Preparation of Citric Acid-Locust Bean Gum (CA-LBG) for the Disintegrating Agent of Tablet Dosage Forms. J Pharm Innov 17, 1160–1175 (2022). https://doi.org/10.1007/s12247-021-09591-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09591-0