Abstract
Background
The primary aim was to investigate outcome of the decision making on duration of injection intervals between injection visits over the first 2 years of a treat and extend regimen.
Method
Consecutive patients receiving Aflibercept for treatment naïve neovascular age-related macular degeneration between 01.01.2016 and 15.07.2017 were identified from our departmental register. Retrospective data collected on all visits over 24 months were classified into three groups: (A) Without Interval Decision Events (IDE)” Injection only” (B) IDE resulting in injection intervals of <5 weeks and (C) IDE resulting in intervals of >5 weeks. The primary outcome was number of successful IDE relative to the total visits in Group C. Successful decision making was defined as absence of worsening of visual acuity (>5 L) or central retinal thickness (>50 microns) at the subsequent visit. Secondary visual and anatomical outcomes at 24 months were also evaluated.
Results
Data from 56 eyes of 50 patients were included in the study. Visual acuity improved by +7.11 L at 24 months. Forty one patients with unilateral therapy made 721 visits: 280 visits (38.8%) were group A; 164 visits (22.8%) were group B and 277 visits (38.4%) were group C. Average interval in Group C was 8.9 weeks (range 5–15). The success rate of extension was 95.31% (264/277 visits).
Conclusion
These metrics for evaluating the decision making aspect of disease activity monitoring may be useful for monitoring performance and have given us a more realistic view and expectations of what can be achieved using this regime to optimise the timing of injections.
Similar content being viewed by others
Introduction
Intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents is the therapy of choice for patients with neovascular age related macular degeneration (nAMD) [1,2,3]. In recent years, repeated intravitreal injections administered on a ‘treat and extend’ (TREX) regimen have gained more popularity [4,5,6,7]. Unlike the pro-re-nata approach, the TREX regimen involves administering injections at every visit but the interval until the next injection is extended or reduced depending on disease activity [8, 9]. This ‘titration’ of injection frequency therefore has the effect of optimising the dosing frequency so that patients who are more stable can have fewer injections over time [10, 11].
Disease activity monitoring is usually performed on the same day as the injection visit based on a combination of factors including visual acuity, biomicroscopy findings and OCT scan findings. Even within the definition of TREX there is variation in the way it is administered and the strictness with which treatment decisions are made. Some clinicians advocate a return to 4 weekly intervals after failing to achieve extension a certain number of times [12]. Others suggest a reduction in treatment interval when an extension has failed [13, 14]. Previous studies have focussed on conventional outcome measures such as visual acuity gain or number of injections given and reported some excellent results both in clinical trial and real-world settings [15, 16] but the variable and subjective nature of decision making on disease activity especially in a large department can potentially lead to suboptimal therapy and outcomes.
The purpose of this study was to evaluate, in a representative cohort of patients, the proportion of their visits where a decision was made on the interval until the next injection and how frequently there was a worsening of disease activity whenever the interval decision event (IDE) resulted in an interval of more than 5 weeks. These two aspects of the TREX regimen are probably the most relevant to patients as they determine how likely they are to enjoy injection free intervals of more than 5 weeks and how likely these longer intervals will result in recurrence of disease activity. They may also be useful tools for providing a standard approach for comparing performance, for instance, before and after an implemented systematic change in the disease activity definition or protocol for retreatment. These metrics may also provide an additional insight into the burden of implementing a treat and extend regimen and provide novel benchmarks on the performance of a departmental team in making decisions about treatment intervals.
Method
Consecutive patients who were commenced on aflibercept on a treat and extend regimen between 1st January 2016 and 15th July 2017 for treatment naïve nAMD in one or both eyes were identified from our hospital electronic database. This study entry period was selected because, by that time, there was a well-established, senior nurse and optometrist-led, decision making process for patients undergoing intravitreal therapy for nAMD using the treat and extend (TREX) regimen in our hospital department. The time scale also provided sufficient sample size of patients who would have completed at least 24 months of follow up. In our departmental nAMD service, disease activity assessment and decision making on the interval between injections would have been made by allied health professional staff. An IDE would normally involve consideration of several factors including habitual visual acuity on ETDRS chart (VA), lesion appearance of OCT scan and other factors such as fellow eye status or patients’ social circumstances. Case notes were reviewed on our hospital portal system and those patients who did not meet the inclusion criteria were excluded and the reasons logged. Data on VA, central retinal thickness (CRT), and presence of any co-existing sight affecting pathology at every visit were collected from hospital records on all remaining eligible patients between July 2019 and November 2019. The proportion of visits that resulted in an injection event was also used to evaluate how strictly the TREX regimen was adhered to. The departmental policy would have been to administer injections every month until dryness or stability was achieved on OCT scan. At any visit after the loading phase, a decision should have been made on whether to extend the interval by increments of 2 weeks at a time if there was stable or no disease activity in accordance with the drug label. Any visit with a decision of persistent or recurrence activity would have triggered a reduction in interval of 4 or 5 weeks. Any visit with a decision of stability would have triggered an extended interval of more than 4 or 5 weeks. Our main interest was to evaluate the success of decision making which involved those extended intervals longer than 5 weeks. To do this, visits were classified into: (A) without an IDE (injection only); (B) with an IDE resulting in an interval of <5 weeks and (C), with an IDE resulting in an interval of ≥5 weeks.
The primary analysis was on visits and the secondary analysis was on clinical outcomes. Firstly, the proportion of visits that had an IDE resulting in an interval of >5weeks (Group C) was calculated. Then, the success rate of decision making at these visits was evaluated by looking at VA and CRT parameters at the subsequent visit. An unsuccessful decision was defined as a worsening of VA by >5 letters, or a worsening of CRT by >50 microns at the subsequent visit. Analysis was performed based on each of these criteria separately and then with the VA and CRT criteria combined in case VA reduction occurred due to other conditions such as cataract or macular atrophy. For this primary outcome analysis to evaluate the success of decision making when intervals were longer than 5 weeks, we only used patients who had unilateral injections to avoid the confounding effect of the common practice of slightly lengthening or shortening an interval with the specific intention to synchronise both eyes for injection on the same visit in patients undergoing bilateral injections. For the same reason, the number of injections (min, max, arithmetic mean) over the two-year period was also calculated from patients who had unilateral treatment.
However, for secondary outcome analysis of visual and anatomical performance of the TREX regimen that was achieved in our department, we used the data set from unilaterally and bilaterally treated patients over 24 months.
Results
A total of 76 patients were commenced on treatment for treatment-naïve nAMD during the study entry period. Twenty-six patients (31 eyes) were excluded from primary and secondary analyses because of the following reasons: conversion to PRN regimen before month 24 (9 eyes), treated with ranibizumab (4 eyes), receiving injections prior to study entry period (3 eyes) and insufficient follow-up (15 eyes). The reasons for the 15 eyes of 15 patients with insufficient follow up were: death before 24 months (9 patients), patients stopped attending (4 patients), stopping treatment at month 15 due to futility (1 patient), and early discontinuation due to bilateral geographic atrophy (1 patient).
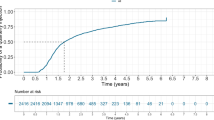
Of the remaining 50 patients, 60% were female (n = 30) and 40% were male (n = 20). The mean age of patient was 78.36 years (range 63–93 years). The 56 eyes of these 50 patients had predominantly occult lesions in 25 eyes (44.6%), retinal angiomatous proliferation (RAP) lesions in 16 eyes (28.6%), classic lesions in 8 eyes (14.3%) and uncertain lesion type in 1 eye (1.8%). Out of these 50 patients, 41 patients had unilateral and complete treat and extend regimen without compromise. Data from this subgroup of 41 patients were therefore used for the primary outcome analysis to evaluate the success rate of decision making. For the secondary anatomical outcome, we used data from all 50 patients of whom 9 had bilateral injections (56 eyes in total). One of these 56 eyes had visual loss due to severe cataract and another due to advanced geographic atrophy. Secondary visual outcome was therefore analysed on 55 eyes of 50 patients (see Fig. 1). These 50 patients made 18.02 visits on average over the 2 years follow up period, with a range of 11 to 25 visits and received between 8 to 25 (mean 17.02) injections over the 2-year period. Over the two-year period, 24 eyes (42.9%) received 20 to 25 injections, 16 eyes (28.6%) received 14 to 19 injections, and 16 eyes (28.6%) received 8 to 13 injections. In keeping with the one-stop, treat and extend regimen, patients in the primary outcome group (n = 41) made 721 visits in total and received injections in 95.70% of all visits.
Only 4.3% of visits involved two stop injections. This would have been due to lack of injectors on the day and a second visit had to be arranged for the injection. This rescheduled injection visits usually occurred within a one-week window. For this analysis, the length of the interval was taken from the day the interval decision event was made and for 95.7% of patients, this was the same day as the injection.
Out of 721 visits: at 280 visits (38.8%), there was no decision made whether to extend treatment (i.e. Group A visits when patients were attending for injection only without reassessment); at 164 visits (22.8%), there was a decision made either to not extend the treatment interval beyond 5 weeks, maintain an interval of <5 weeks or reduce the interval to <5 weeks and therefore these visits were followed by an interval of <5 weeks (Group B visits); at 277 visits (38.4%), there was a decision made that resulted in an interval of >5 week (Group C visits). On average Group C visits were followed by an interval of 8.9 weeks (range 5–15 weeks). Of the 277 visits where a decision was made for the treatment interval to be >5weeks, the interval was 5–7 weeks at 103 visits (short intervals), 8–10 weeks at 85 visits (medium intervals) and more than 11 weeks at 89 visits (long intervals) (Table 1).
Success of interval decision events
Firstly, using the definition of a successful decision based on having a stable CRT only, 264 out of 277 (95.31%) visits were successful. Success rates were similar regardless of the length of intervals.
Secondly, using the definition of a successful decision based on having a stable or improved CRT and stable or improved VA at reassessment, 241 out of 277 visits (87.00%) were successful and 36 decisions were followed by a worsening of CRT by >50 microns or worsening in VA of >5 letters. Of these 36 ‘failed’ decisions, 13 failed because CRT had worsened by >50 microns and 23 failed because BCVA had worsened by >5 letters. Overall, success rate was still high regardless of the length of the interval but slightly lower in the longer interval groups. (Table 1)
Secondary outcomes
The mean baseline CRT was 402.25 microns (range of 205 to 694 microns). The mean CRT at 24 months was 279.59 microns (range 165 to 423 microns). From baseline to 24 months, there was an average reduction in CRT of 122.66 microns (range −398 to +46 microns). Fifty two out of 56 eyes (92.9%) had improved or stable CRT at 24 months compared to baseline. Four eyes (7.1%) had increased CRT at 24 months compared to baseline. The mean baseline VA was 57.27 letters (range 30 to 85 letters). The mean VA at 24 months was 62.95 letters (range 0 to 85 letters). From baseline to 24 months, eyes had an average improvement of +7.11 letters (range −55 to +43 letters). Forty five out of 55 eyes (81.8%) had improved or stable BCVA at 24 months, compared to baseline. Ten eyes (18.2%) had at >5 letters loss at 24 months compared to baseline. Of these 10, 6 eyes (10.9%) lost between 6 and 15 letters and 4 (7.3%) lost >15 letters. At baseline, 14.5% (n = 8) of eyes had more than 70 letters 81.8% (n = 44) of eyes had between 35 and 70 letters and 3.6% (n = 2) had worse than 35 letters. At 24 months, 41.8% (n = 23) of eyes had more than 70 letters, 50.9% (n = 28) of eyes had between 35 and 70 letters and 7.3% (n = 4) had worse than 35 letters (Table 2).
Discussion
The TREX anti-VEGF regimen has gained popularity over recent years for the management of nAMD and impressive visual outcomes have been reported both in clinical trial settings and in real world studies. Clinical reports and presentations of cases showcasing the advantages of the TREX regimen tend to exemplify patients that have frequent successful extensions in their treatment intervals [17, 18]. In this study, we found that only 38.4% of all visits resulted in an IDE followed by an interval of >5 weeks There are no published studies that have reported on the proportion of visits that result in an extended interval as a specific outcome, but a closely related metric is one that describes proportion of patients who achieve an extended duration at least once, at any point in the study. The ATLAS trial reported 37.5% of patients were stable at all visits and 38% of all patients achieved extensions up to 12 weeks or longer [18]. In the original VIEW trials, a post hoc analysis revealed that up to 53.7% of patients achieved a treatment interval of >12 weeks at some stage during the study and maintained stable acuity and retinal thickness as a group but the success or failure of these extended intervals was not evaluated [19]. A recent prospective study of Japanese patients, however reported that out of 97 eyes, 25 eyes had maintained no disease activity immediately after the loading phase and maintained successful extension to an injection interval of 3 months [20]. In the real-world setting, the FRB study by Arnold et al. using predominantly ranibizumab on a treat and extend basis reported 21% to 29% of patients being treated every 9 to 15 weeks in the real world with 83% of visits associated with an injection (i.e., quite a strict one-stop, TREX regimen) [21, 22]. Comparably, our study also had 95.7% of visits associated with injections. Therefore, from our findings and indirect evidence from published literature, it is reasonable to expect that less than 50% of visits can be followed by an interval longer than 4 or 5 weeks in the first two years of a TREX regimen for nAMD.
It was reassuring however to find that in those visits that actually involved an IDE that resulted in an interval of >5 weeks (Group C), we found a good success rate, either when we used the definition based on OCT parameter only (95.31%) or using the definition based on both OCT and VA parameters (87.0%). We believe this was an important finding as good performance on this metric provides evidence of sufficient decision making skills within our department to interpret OCT scans and provide a reliable disease activity monitoring service. It was also encouraging to see that about one third of all visits involved longer intervals of at least 11 weeks and the success rates of decision interval events were very similar regardless of whether they were short, medium or longer intervals. This provides further evidence that the same criteria for decision making can be used successfully regardless of the interval involved.
In this study we found a mean of 17 injections in 24 months. This is similar to those prospective studies using retreatment protocols which recommended a hard return to 4 weekly intervals at the slightest sign of activity but other real-world studies which did not mandate such a hard return to 4 weekly (reducing interval by two weeks instead) have reported lower injection frequency [23,24,25,26] Several factors may have contributed to our higher frequency of injections. The high number of injections seen in our study could be explained by, our then, low tolerance for residual fluid and the tendency to repeat loading at ‘injection only’ visits. In cases where it was felt that a patient was unlikely to stabilise adequately to allow an extension at the next visit, they would be listed for two or three injections in a loop, often at monthly intervals in an attempt to ‘stabilise’ an unstable macula before considering the next extension. Therefore, the low proportion of visits that that led to an extended interval of >5 weeks (38.4%) and the high proportion of ‘injection only’ visits (38.8%) found in our study are both inter-related metrics that can be explained by the effect of our tendency to perform repeated loading loops which in turn resulted in fewer opportunities to make decisions to extend visit intervals. In theory, within a 24-month period, there should be 2 visits that did not need an IDE followed typically by about 15 visits in which there is a decision made to extend or not to extend/reduce the interval. Therefore, the benchmark value of this metric should be in the region of 12%. Therefore, this metric can only be improved by not attempting to do repeated loading doses at fixed intervals of every 4 to 8 weeks but to perform OCT scans at every visit and to make a decision on disease activity from and including the third injection visit onwards. This however puts a heavier burden on staff resources especially for performing and interpreting OCT scans. It is because of this reason that we had such a poor performance on this metric in our department. Secondly, we only included patients who remained on TREX regimen throughout the 24-month study period. Seven patients were switched to PRN regimen at an early stage because of good response after only a small number of initial injections. This would have had the effect of selecting a group with more aggressive nAMD that remained on TREX. This factor is often not considered when we compare injection rates between various studies using TREX regimen in the real world. Another factor that contributed to a high frequency of injections was our tendency to retreat every month to attempt to ‘dry up’ the neovascular lesion. Since this study, we have implemented a more tolerant approach to subretinal fluid. We have done this by highlighting to our staff the finding of relevant studies that support tolerating some fluid and also the results of the pivotal CATT study which showed that 51.5% of all patients had fluid on OCT after monthly injections of ranibizumab for 24 months [27, 28].
Our visual acuity gain metric was +7.11 letters at 24 months. This is comparable to other similar real-world studies [29, 30]. In a retrospective study of 231 patients over 4 years, Traine et al. [30] reported a visual gain of 5.7 letters at 24 months. Their attrition rate of 25% over two years was similar to ours (20%). This also highlights the issue of applicability of the TREX regimen. In addition to natural attrition due to death, the futility factor, which can cause a degree of attrition over time in any treated cohort, a significant proportion of patients who do well can also be switched to a PRN regiment. We found about 13% of patients were switched to a less vigorous treatment regimen of PRN or Monitor and Extend. This makes comparison of different TREX studies difficult in terms of visual outcomes.
Another finding worth noting was our proportion of bilaterally affected patients. We had a point prevalence of 11 out of 76 patients (14.5%) with bilateral injections during the study period. As it is common practice to try and synchronise visits for both eyes, we have excluded these patients from our analysis of decision-making data. With such patients there is always a balance and compromise between optimal dosing for each eye with the logistics of unsynchronised and frequent visits for unilateral injections versus the sub-optimal treatment of one eye to reduce burden of visits. With numbers of patients requiring injections for nAMD increasing every year, it is anticipated that the numbers of patients requiring bilateral injections will also increase proportionately and therefore the goal of optimising therapy for each eye of every patient can be expected to become more challenging.
In summary, we have performed a visit-by-visit analysis to evaluate the frequency and success of decisions we made on the duration of the injection free interval for a representative cohort of patients undergoing the first two years of nAMD therapy. We found that whenever decisions were made to give patients a longer interval between injections, there was a very low risk of worsening disease activity, but we also found that the frequency of extended intervals was less than our preconceived expectations. In this report we have hypothesised the possible reasons for these findings and provided opinions on how to improve on these metrics. We believe these novel metrics for evaluating the decision-making aspect of disease activity monitoring will be especially useful for monitoring performance in future particularly when there is a change in the criteria used for judging disease activity. Finally, our findings have given us a more realistic view and expectations of what can be achieved using the TREX regimen in terms of the interval between injections and this will be useful for us and other clinicians to improve how we counsel and reassure patients.
Summary table
What is known about this topic:
-
Treat and extend regime of anti VEGF therapy for neovascular AMD has been accepted universally by treating physicians as a menthod for optimising disease control and visual outcomes for suitable patients.
-
It has the potential for reducing burden of frequent visits and injections both for the patients and the treating unit.
What this study adds:
-
Classifying the injection visits into three categories based on the type of desicion made on injection interval.
-
Creating a novel metrics for measuring the success of the desicion making to extend the injection interval beyond 5 weeks. The metrics may be a useful tool for providing a standard approach for comparing performance and provide an additional insight into the burden of implementing a treat and extend regimen.
References
Kovach JL, Schwartz, SG, Flynn, HW, Jr, & Scott, IU. (2012). Anti-VEGF Treatment Strategies for Wet AMD. Journal of ophthalmology, 2012:786870. https://doi.org/10.1155/2012/786870.
Villegas VM, Aranguren LA, Kovach JL, Schwartz SG, Flynn HW Jr. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin drug Deliv. 2017;14:273–82.
Ventrice P, Leporini C, Jose’Francisco Aloe EG, Leuzzi G, Marrazzo G, Scorcia GB, et al. Anti-vascular endothelial growth factor drugs safety and efficacy in ophthalmic diseases. J Pharmacol Pharmacother. 2013;4:S38.
Gemenetzi M, Patel PJ. A systematic review of the treat and extend treatment regimen with anti-VEGF agents for neovascular age-related macular degeneration. Ophthalmol Ther. 2017;6:79–92.
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Ng P, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137:372–9.
Okada M, Kandasamy R, Chong EW, McGuiness M, Guymer RH. The treat-and-extend injection regimen versus alternate dosing strategies in age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2018;192:184–97.
Augsburger M, Sarra GM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:1889–95.
Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, Bonicel P, et al. Inject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina 2011;31:26–30.
Wakuta M, Nomi N, Ogata T, Ota M, Yamashiro C, Hatano M, et al. A Trinity regimen with aflibercept for treatment-naïve neovascular age-related macular degeneration: 2-year outcomes. Graefes Arch Clin Exp Ophthalmol. 2020;258:1663.
Abedi F, Wickremasinghe S, Islam AF, Inglis KM, Guymer RH. Anti-VEGF treatment in neovascular age–related macular degeneration: a treat-and-extend protocol over 2 years. Retina 2014;34:1531–8.
Toalster N, Russell M, Paul NG. A 12-month prospective trial of inject and extend regimen for ranibizumab treatment of age-related macular degeneration. Retina 2013;33:1351–8.
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Pecheur FL, et al. Macular atrophy in neovascular age-related macular degeneration: a randomized clinical trial comparing Ranibizumab and Aflibercept (RIVAL Study). Ophthalmology 2020;127:198–210.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. TREX-AMD Study Group. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015;122:2514–22.
Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 2019;126:723–34.
Barthelmes D, Nguyen V, Daien V, Campain A, Walton R, Guymer R, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina 2018;38:20–8.
Tsunekawa Y, Kataoka K, Asai K, Ito Y, Terasaki H. Four-year outcome of aflibercept administration using a treat-and-extend regimen in eyes with recurrent neovascular age-related macular degeneration. Jpn J Ophthalmol. 2021;65:69–76. https://doi.org/10.1007/s10384-020-00783-8.
Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015;122:146–52.
DeCroos FC, Reed D, Adam MK, Salz D, Gupta OP, Ho AC, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142–50.
Khurana RN, Rahimy E, Joseph WA, Saroj N, Gibson A, Vitti R, et al. Extended (every 12 weeks or longer) dosing interval with intravitreal aflibercept and ranibizumab in neovascular age-related macular degeneration: post hoc analysis of VIEW trials. Am J Ophthalmol. 2019;200:161–8.
Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, et al. Two-Year Outcomes of Treat-and-Extend Intravitreal Aflibercept for Exudative Age-Related Macular Degeneration: A Prospective Study. Ophthalmol Retina. 2020;4:767–76. https://doi.org/10.1016/j.oret.2020.03.010.
Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP, et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology 2015;122:1212–9.
Bhandari S, Nguyen V, Arnold J, Young S, Banerjee G, Gillies M, et al. Treatment outcomes of Ranibizumab versus Aflibercept for neovascular age-related macular degeneration: data from the Fight Retinal Blindness! Registry. Ophthalmology 2020;127:369–76.
Mekjavić PJ, Gregorčič B, Oberč C, Podgoršek S. Treat-and-extend therapy using intravitreal aflibercept for neovascular age-related macular degeneration: 2-year real-world practice data from Slovenia. BMC Ophthalmol. 2018;18:1–7.
Taipale C, Lindholm JM, Kaarniranta K, Tuuminen R. Comparison of two different treat-and-extend protocols with aflibercept in wet age-related macular degeneration: two-year results. Adv Ther. 2020;37:2256–66.
Taipale C, Lindholm JM, Laine I, Tuuminen R. Comparison of two different treat‐and‐extend protocols with aflibercept in wet age‐related macular degeneration. Acta Ophthalmol. 2020;98:267–73.
Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration-a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016;16:1–9.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Comparison of age-related macular degeneration Treatments Trials (CATT) Research group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388–98.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Comparison of age-related macular degeneration Treatments Trials (CATT) Research group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2020;127:S135–45.
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52-and 96-week findings from ALTAIR. Adv Ther. 2020;37:1173–87.
Traine PG, Pfister IB, Zandi S, Spindler J, Garweg JG. Long-term outcome of intravitreal aflibercept treatment for neovascular age-related macular degeneration using a “treat-and-extend” regimen. Ophthalmol Retin. 2019;3:393–9.
Author information
Authors and Affiliations
Contributions
BMcL: Data collection and contribution towards manuscript writing. AM: Data collection and contribution towards manuscript writing. MK: Contribution towards manuscript writing and corresponding author. BT: Data collection and contribution towards manuscript writing. NN: Data analysis and guidance for manuscript writing. YY: Project lead, Data analysis, manuscript writing and guidance for manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McLeish, B., Morris, A., Karpoor, M. et al. Novel metrics for evaluating decision making in a ‘Treat and Extend’ regimen for neovascular age related macular degeneration. Eye 36, 1994–1999 (2022). https://doi.org/10.1038/s41433-021-01785-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01785-7