Abstract
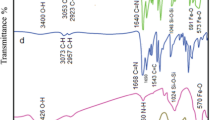
In this research, magnetite nanoparticles as the core are fabricated by co-precipitation method from Fe(II) and Fe(III) chloride salts and then the surfaces of the nanoparticles are modified to improve their performance. These modifications include the coating of silica and then the second layer by 3-amino propyl triethoxy silane (APTES) which causes the functionalization of magnetite nanoparticles with –NH2 groups. In the next step, an aliphatic tetra-dentate N2O2 Schiff base was synthesized separately by condensation reaction between benzoyl acetone and ethylenediamine. This free Schiff base has two C = O groups. By the condensation reaction between one C = O group of free Schiff base and –NH2 group of functionalized magnetite silica, a tetra-dentate N3O Schiff base is immobilized on the magnetite silica surface. This tetra-dentate Schiff base supported on magnetite silica is a very good structure for the formation of stable metal complexes. The immobilized Schiff base was converted to the Ni(II) Schiff base complex by the reaction with Ni(II) acetate tetra-hydrate. This Ni(II) Schiff base complex is immobilized on magnetite silica and shown as Fe3O4@SiO2/Schiff base of Ni(II). This core-shell nanostructure is fully characterized by techniques such as FT-IR, VSM, XRD, FE-SEM, EDX, TGA-DTA, AAS, BET, and BJH. In the last step, this nanocatalyst is used as an efficient catalyst for the solvent-free synthesis of amido alkyl naphthols by the condensation reaction between an aldehyde, 2-naphthol, and an amide. The reaction was monitored by TLC. At the end of the reaction, the nanocatalyst was removed easily from the reaction mixture by an external magnet. The products of 1-amidoalkyl-2-naphthols were identified by FT-IR and 1HNMR techniques. Finally, the percentage yield of the reaction is calculated by measuring the mass of 1-amidoalkyl-2-naphthol synthesized in the presence of the catalyst.

















Similar content being viewed by others
References
K. Kannan, D. Radhika, K.K. Sadasivuni, K.R. Reddy, A.V. Raghu, Adv. Colloid Interface Sci. 281, 102178 (2020)
M. Ghanbari, S. Moradi, M. Setoodehkhah, Green Chem. Lett. Rev. 11, 111 (2018)
J.L. Hodala, D.J. Moon, K.R. Reddy, C.V. Reddy, T.N. Kumar, M.I. Ahamed, A.V. Raghu, Int. J. Hydrog. Energy 46, 3289 (2021)
K. Kannan, D. Radhika, A.S. Nesaraj, K.K. Sadasivuni, K.R. Reddy, D. Kasai, A.V. Raghu, Mater. Sci. Technol. 3, 853 (2020)
K. Kannan, D. Radhika, K.R. Reddy, A.V. Raghu, K.K. Sadasivuni, G. Palani, K. Gurushankar, Nano Express 2, 010014 (2021)
M. Srinivas, R.C. Venkata, R.R. Kakarla, N.P. Shetti, M.S. Reddy, V.R. Anjanapura, Mater. Res. Express 6, 125502 (2019)
Z. Karimi-Jaberi, M.S. Moaddeli, M. Setoodehkhah, M.R. Nazarifar, Res. Chem. Intermed. 42, 4641 (2016)
X. Li, S. Zhang, B. Yang, C. Lv, X. Jia, Z. Hu, RSC Adv. 6, 86531 (2016)
A. Azizi, J. Inorg. Organomet. Polym Mater. 30, 3552 (2020)
A. Emadi, A. Feizbakhsh, A. Niazi, J. Inorg. Organomet. Polym Mater. (2020). https://doi.org/10.1007/s10904-020-01594-7
H. Nosrati, N. Rashidi, H. Danafar, H.K. Manjili, J. Inorg. Organomet. Polym Mater. 29, 2292 (2019)
S. Karimi, H. Namazi, New J. Chem. 45, 6397 (2021)
A. Sajjadi, R. Mohammadi, J. Med. Chem. Sci. 2, 55 (2019)
Q.-L. Zhu, Q. Xu, Chem. 1, 220 (2016)
J. Liu, H. Qiu, F. Zhang, Y. Li, New J. Chem. 44, 5324 (2020)
N. Amirmahani, H. Mahdizadeh, M. Malakootian, A. Pardakhty, N. Mahmoodi, J Inorg. Organomet. Polym. Mater. 30, 3540 (2020)
S. Maria, M. Georgescu, L. Alexandrescu, D. Gurau, A. Ficai, D. Ficai, E. Andronescu, Curr. Pharm. Des. 21, 5324 (2015)
M. Langeroudi, M. Pourabhari, E. Binaeian, Mater. Chem. Phys. 218, 210 (2018)
H. Rajabi, H. Arjmand, S.J. Hoseini, H. Nasrabadi, Magn. Mater. 394, 7 (2015)
A. Behbahani, N. Sadati, K. Rostamizadeh, M.R. Yaftian, A. Zamani, H. Ahmadi, J. Environ. Health. Sci. 12, 1 (2014)
A. Araghi, S. Hozhabr, M.H. Entezari, Appl. Surf. Sci. 333, 68 (2015)
M. Asadi, M. Setoodehkhah, A.H. Kianfar, J. Iran. Chem. Soc. 7, 38 (2010)
L. Xiang, J.R. Hamon, Coord. Chem. Rev. 389, 94 (2019)
K.C. Gupta, A.K. Sutar, Coord. Chem. Rev. 252, 1420 (2008)
K. Mohammadi, M. Asadi, M. Setoodehkhah, H. Sepehrpour, Croat. Chem. Acta 89, 277 (2016)
Z. Asadi, M. Asadi, M. Setoodehkhah, Spectrochim. Acta, A, Mol. Biomol. Spec. 112, 214 (2013)
M. Asadi, M. Setoodehkhah, J. Iran. Chem. Soc. 8, 875 (2010)
G. Mohamed, Spectrochim. Acta. A. Mol. Biomol. Spec. 64, 188 (2006)
N. Kocak, M. Sahin, G. Arslan, H.I. Ucan, J. Inorg. Organomet. Polym. 22, 166 (2012)
D. Kara, A. Fisher, S.J. Hill, J. Hazard. Mater. 165, 1165 (2009)
M. Setoodehkhah, S. Momeni, J. Inorg. Organomet. Polym. 28, 1098 (2018)
A. Afkhami, T. Madrakian, R. Ahmadi, H. Bagheri, M. Tabatabaee, Microchim. Acta 175, 69 (2011)
H.R. Shaterian, H. Yarahmadi, Tetrahedron Lett. 49, 1297 (2008)
A.R. Hajipour, Y. Ghayeb, N. Sheikhan, A.E. Ruoho, Tetrahedron Lett. 50, 5649 (2009)
A.E. Rosamilia, C.R. Strauss, J.L. Scott, Pure Appl. Chem. 79, 1869 (2007)
S. Rajesh, R. Bala, R. Duvedi, S. Kumar, Iran. J. Catal. 5, 187 (2015)
H.R. Shaterian, A. Amirzadeh, F. Khorami, M. Ghashang, Synth. Commun. 38, 2983 (2008)
G.C. Nadi, S. Samai, R. Kumar, M.S. Singh, Tetrahedron. Lett. 50, 7220 (2009)
B. Baghernejad, E. Ashoori, J. Appl. Chem. Res. 14, 22 (2021)
D. Afinisha, J. Viswanadhan, Orient. J. Chem. 33, 1354 (2017)
H. Kiyani, H. Darbandi, A. Mosallanezhad, F. Ghorbani, Res. Chem. Intermed. 4, 7561 (2015)
R. Ghorbani-Vaghei, S. Malaekehpour, Open Chem. J. 8, 1086 (2010)
D. Biswanath, C.R. Reddy, J. Kashanna, S.K. Mamidyala, C.G. Kumar, Med. Chem. Res. 21, 3321 (2012)
B. Khawla, H. Boulebd, C. Bensouici, D. Harakat, R. Boulcina, A. Debache, Chem. Select. 5, 5515 (2020)
B. Bananezhad, M.R. Islami, H. Khabazzadeh, J. Iran. Chem. Soc. 16, 865 (2019)
W. Min, Z. Song, Y. Liang, Org. Prep. Proced. Int. 43, 484 (2011)
B. Maleki, F. Taimazi, Org. Prep. Proced. Int. 46, 252 (2014)
N. Lingaiah, M. Baseeruddin, S. Apuri, S. Kantevari, Catal. Commun 8, 1729 (2007)
S.A. Ansari, M.K. Jaiprakash, N. Sangshetti, N.D. Kokare, P.S. Wakte, D.B. Shinde, Ind. J. Chem. Technol. 17, 71 (2010)
R. Tayebee, M. Amini, M. Akbari, A. Aliakbari, Dalton Trans. 44, 9596 (2015)
M. Esmaeilpour, J. Javidi, M. Zandi, Mater. Res. Bull 55, 78 (2014)
Z. Cai, C. Shu, Y. Peng, Monatsh. Chem. 145, 1681 (2014)
D. Katheriya, N. Patel, H. Dadhania, A. Dadhania, J. Iran. Chem. Soc. 18, 805 (2021)
J. Safari, Z. Zarnegar, J. Mol. Catal. A Chem. 379, 269 (2013)
G. Kafili, M.R. Loghman-Estarki, M. Milani, B. Movahedi, J. Am. Ceram. Soc. 100, 4305 (2017)
F. Davar, H. Hadadzadeh, T.S. Alaedini, Ceram. Int. 42, 19336 (2016)
A. Noormohamadi, M. Homayoonfal, M.R. Mehrnia, F. Davar, Ceram. Int. 43, 17174 (2017)
M. Assefi, F. Davar, H. Hadadzadeh, Adv. Powder Technol. 26, 1583 (2015)
M. Tumer, C. Celik, H. Koksal, S. Serin, Trans. Met. Chem. 24, 525 (1999)
M.R. Loghman-Estarki, S. Torkian, R.A. Rastabi, A. Ghasemi, J. Magn. Magn. Mater. 442, 163 (2017)
W. Wang, P. Liu, M. Zhang, J. Hu, F. Xiang, Open J. Compos. Mater. 2, 104 (2012)
L. Nagarapu, M. Baseeruddin, S. Apuri, S. Kantevari, Indian J Chem. Technol. 312, 53 (2007)
H.R. Shaterian, H. Yarahmadi, M. Ghashang, Tetrahedron 64, 1263 (2008)
M. Dehbashi, M. Aliahmad, M. Shafiee, M. Ghashang, Inorg. Nano-Met. Chem. 43, 1301 (2013)
S. Bahrami, S. Jamehbozorgi, S. Moradi, S. Ebrahimi, J Chin. chem. Soc. 67, 603 (2020)
Q. Zhang, Y.H. Gao, S.L. Qin, H.X. Wei, Catalysts 7, 351 (2017)
Acknowledgements
The authors are grateful to University of Kashan for supporting this work.
Funding
This study was funded by University of Kashan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazraati, A., Setoodehkhah, M. & Moradian, M. Synthesis of Bis (Benzoyl Acetone Ethylene Diimine) Schiff Base Complex of Nickel (II) Supported on Magnetite Silica Nanoparticles (Fe3O4@SiO2/Schiff-Base of Ni(II)) and Using It as an Efficient Catalyst for Green Synthesis of 1-Amidoalkyl-2-Naphthols. J Inorg Organomet Polym 32, 143–160 (2022). https://doi.org/10.1007/s10904-021-02119-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02119-6