Abstract
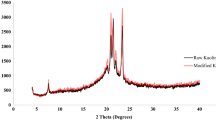
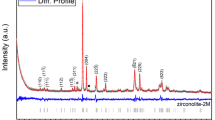
The intercalation of kaolinite through the insertion of ions or molecules amongst the structural aluminosilicate layers is a vital process in numerous clay-based applications and products. Layer neutrality and hydrogen bonding limits direct intercalation into kaolinite, other than for small molecules. Synthesizing zirconia-intercalated kaolinite is not a straightforward matter. To overcome this barrier, raw Egyptian kaolin (UnK) or its acid-activated product (HK) was sonicated and impregnated in aqueous ZrOCl2·8H2O solution followed by thermal treatment at various temperatures (100, 200, 300, and 500°C). The intercalation process was confirmed using various spectroscopic and analytical techniques. The direct intercalation of ZrO2 into the kaolinite layers was observed even through a mild thermal treatment (100, 200, and 300°C). The mechanism of intercalation was suggested to occur by binding ZrO2 to the Si/AlO groups with a preference for the acid-activated HK, causing variable enlargements of the basal spacing and producing very perturbed layers. Interestingly, the surface area increased by 250% as a result of zirconia intercalation. Scanning electron microscopy (SEM) images showed a remarkable improvement in the stacking order of the kaolinite particles. The impact of ZrO2 intercalation into kaolinite also enhanced its adsorption efficiency for Pb2+, Cu2+, and Cd2+ ions. Preliminary investigations showed that the zirconia-intercalated HK demonstrated a removal efficiency, which is three times greater than that of pristine HK. The adsorption tendency toward Pb2+ ions was greater than those of Cu2+ and Cd2+ and followed the order: Pb2+ >> Cu2+ > Cd2+. The study suggests that the chemical modification of kaolin by zirconia via a direct intercalation technique, which greatly improves its functionality as demonstrated by the selective sorption of heavy metal ions, is worthy of further study.









Similar content being viewed by others
REFERENCES
Abou-El-Sherbini, K. S., Elzahany, E. A. M., Wahba, M. A., Drweesh, S. A., & Youssef, N. S. (2017). Evaluation of some intercalation methods of dimethylsulphoxide onto HCl-treated and untreated Egyptian kaolinite. Applied Clay Science, 137, 33–42.
Agarkov, D., Burmistrov, I., Tsybrov, F., Tartakovskii, I., Kharton, V., & Bredikhin, S. (2018). In-situ Raman spectroscopy analysis of the interface between ceria-containing SOFC anode and stabilized zirconia electrolyte. Solid State Ionics, 319, 125–129.
Bailey, S. W. (1966). The status of clay mineral structures. Pp. 1–23 in Clays and Clay Minerals Proceedings of the Fourteenth National Conference, Berkeley, California (S. W. Bailey, editor). Pergamon.
Baird, R. B., Eaton, A. D., & Rice, E. W. (2017). Standard Methods for the Examination of Water and Wastewater. (23rd edition). American Water Works Association; Water Pollution Control Federation; Water Environment Federation, Washington.
Barrett, E. P., Joyner, L. G., & Halenda, P. P. (1951). The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. Journal of the American Chemical Society, 73, 373–380.
Beden, B., & Guillaum, I. (1969). Thermal decomposition of zirconyl chloride octahydrate in ambient air. Comptes rendus hebdomadaires des seances de l academie des sciences series c, 269, 1629–1669.
Berger, M., & Hubbell, J. (1998). 1999 Photon Attenuation Coefficients CRC Handbook of Chemistry and Physics 79th edn, (D R. Lide, editor). CRC Press, Boca Raton, Florida, USA.
Bhattacharyya, K. G., & Gupta, S. S. (2006). Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu (II) from aqueous solution. Separation and Purification Technology, 50, 388–397.
Bhattacharyya, K. G., & Gupta, S. S. (2008). Adsorption of Fe(III), Co(II) and Ni(II) on ZrO-kaolinite and ZrO-montmorillonite surfaces in aqueous medium. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 317, 71–79.
Brunauer, S., Emmett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60, 309–319.
Chaabene, S. B., Bergaoui, L., & Ghorbel, A. (2004). Zirconium and sulfated zirconium pillared clays: a combined intercalation solution study and solid characterization. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 251, 109–115.
Chen, S. B., Ma, Y. B., Chen, L., & Xian, K. (2010). Adsorption of aqueous Cd(II), Pb(II), Cu(II) ions by nano-hydroxyapatite: single-and multi-metal competitive adsorption study. Geochemical Journal, 44, 233–239.
Cheng, H., Liu, Q., Cui, X., Zhang, Q., Zhang, Z., & Frost, R. L. (2012). Mechanism of dehydroxylation temperature decrease and high temperature phase transition of coal-bearing strata kaolinite intercalated by potassium acetate. Journal of Colloid and Interface Science, 376, 47–56.
Chevalier, J., Gremillard, L., Virkar, A. V., & Clarke, D. R. (2009). The tetragonal-monoclinic transformation in zirconia: lessons learned and future trends. Journal of the American Ceramic Society, 92, 1901–1920.
Dedzo, G. K., & Detellier, C. (2016). Functional nanohybrid materials derived from kaolinite. Applied Clay Science, 130, 33–39.
Drweesh, S. A., Fathy, N. A., Wahba, M. A., Hanna, A. A., Akarish, A. I. M., Elzahany, E. A. M., El-Sherif, I. Y., & Abou-El-Sherbini, K. S. (2016). Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. Journal of Environmental Chemical Engineering, 4, 1674–1684.
Farfan-Torres, E., Sham, E., & Grange, P. (1992). Pillared clays: preparation and characterization of zirconium pillared montmorillonite. Catalysis Today,15, 515–526.
Farmer, V. C., & Russell, J. D. (1964). The infra-red spectra of layer silicates. Spectrochimica Acta, 20, 1149–1173.
Frost, R. L., & Vassallo, A. M. (1996). The dehydroxylation of the kaolinite clay minerals using infrared emission spectroscopy. Clays and Clay Minerals, 44, 635–651.
Frost, R. L., Kristof, J., Paroz, G. N., Tran, T. H., & Kloprogge, J. T. (1998). The role of water in the intercalation of kaolinite with potassium acetate. Journal of Colloid and Interface Science, 204, 227–236.
Gao, W., Zhao, S., Wu, H., Deligeer, W., & Asuha, S. (2016). Direct acid activation of kaolinite and its effects on the adsorption of methylene blue. Applied Clay Science, 126, 98–106.
Gil, A., Vicente, M., Lambert, J.-F., & Gandıa, L. (2001). Platinum catalysts supported on Al-pillared clays: Application to the catalytic combustion of acetone and methyl-ethyl-ketone. Catalysis today, 68, 41–51.
Gong, L., Sun, L.-B., Sun, Y.-H., Li, T.-T., & Liu, X.-Q. (2011). Exploring in situ functionalization strategy in a hard template process: Preparation of sodium-modified mesoporous tetragonal zirconia with superbasicity. The Journal of Physical Chemistry C, 115, 11633–11640.
Gorodylova, N., & Šulcová, P. (2018). DTA-TGA and XRD study of the formation of LISICON-type Li1+xCrxZr2−x(PO4)3 ceramic using ZrOCl2·8H2O as precursor. Journal of Thermal Analysis and Calorimetry, 133, 405–411.
Gorodylova, N., Šulcová, P., Bosacka, M., & Filipek, E. (2014). DTA-TG and XRD study on the reaction between ZrOCl2·8H2O and (NH4)2HPO4 for synthesis of ZrP2O7. Journal of Thermal Analysis and Calorimetry, 118, 1095–1100.
Grzybek, T., Klinik, J., Olszewska, D., Papp, H., & Smarzowski, J. (2001). The influence of montmorillonite treatment on structure, sorption properties and catalytic behaviour: Part I. Zirconia pillared clays modified with manganese as Denox catalysts. Polish Journal of Chemistry, 75, 857–868.
Gupta, S. S., & Bhattacharyya, K. G. (2005). Interaction of metal ions with clays: I. A case study with Pb(II). Applied Clay Science, 30, 199–208.
Gupta, S. S., & Bhattacharyya, K. G. (2006). Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly (oxo zirconium) and tetrabutylammonium derivatives. Journal of Hazardous Materials, 128, 247–257.
Gupta, S. S., & Bhattacharyya, K. G. (2008). Immobilization of Pb (II), Cd (II) and Ni (II) ions on kaolinite and montmorillonite surfaces from aqueous medium. Journal of Environmental Management, 87, 46–58.
Ianchis, R., Corobea, M., Donescu, D., Rosca, I., Cinteza, L., Nistor, L., Vasile, E., Marin, A., & Preda, S. (2012). Advanced functionalization of organoclay nanoparticles by silylation and their polystyrene nanocomposites obtained by miniemulsion polymerization. Journal of Nanoparticle Research, 14, 1–12.
Ilić, B. R., Mitrović, A. A., & Miličić, L. R. (2010). Thermal treatment of kaolin clay to obtain metakaolin. Hemijska industrija, 64, 351–356.
Jiang, M.-q., Jin, X.-y., Lu, X.-q., & Chen, Z.-l. (2010). Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination, 252, 33–39.
Jiang, H., Liu, G., Hu, Y., Xu, L., Yu, Y., Xie, Z., & Chen, H. (2013). Flotation and adsorption of quaternary ammonium salts collectors on kaolinite of different particle size. International Journal of Mining Science and Technology, 23, 249–253.
Kenne Dedzo, G., & Detellier, C. (2017). Characterization and Applications of Kaolinite Robustly Grafted by an Ionic Liquid with Naphthyl Functionality. Materials (Basel, Switzerland), 10, 1006.
Kristó, J., Frost, R. L., Felinger, A., & Mink, J. (1997). FTIR spectroscopic study of intercalated kaolinite. Journal of Molecular Structure, 410, 119–122.
Kristóf, T., Sarkadi, Z., Ható, Z., & Rutkai, G. (2018). Simulation study of intercalation complexes of kaolinite with simple amides as primary intercalation reagents. Computational Materials Science, 143, 118–125.
Ledoux, R. L., & White, J. L. (1966). Infrared studies of hydrogen bonding interaction between kaolinite surfaces and intercalated potassium acetate, hydrazine, formamide, and urea. Journal of Colloid and Interface Science, 21, 127–152.
Lee, D. H., & Moon, H. (2001). Adsorption equilibrium of heavy metals on natural zeolites. Korean Journal of Chemical Engineering, 18, 247–256.
Libowitzky, E. (1999). Correlation of O-H stretching frequencies and O-H O hydrogen bond lengths in minerals. Pp. 103–115 in: Hydrogen Bond Research (P. Schuster & W. Mikenda, editors). Springer, Vienna.
Libowitzky, E., & Beran, A. (2004). IR spectroscopic characterisation of hydrous species in minerals. Spectroscopic Methods in Mineralogy, 6, 227–279.
Majd, M. T., Davoudi, M., Ramezanzadeh, M., Ghasemi, E., Ramezanzadeh, B., & Mahdavian, M. (2020). Construction of a smart active/barrieranti-corrosion system based on epoxy-ester/zinc intercalated kaolin nanocontainer for steel substrate. Construction and Building Materials, 247, 118555.
Makó, É., Kovács, A., Katona, R., & Kristóf, T. (2016). Characterization of kaolinite-cetyltrimethylammonium chloride intercalation complex synthesized through eco-friend kaolinite-urea pre-intercalation complex. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 508, 265–273.
Matsui, K., & Ohgai, M. (2002). Formation Mechanism of Hydrous Zirconia Particles Produced by Hydrolysis of ZrOCl2 Solutions: IV, Effects of ZrOCl2 Concentration and Reaction Temperature. Journal of the American Ceramic Society, 85, 545–553.
Miehé-Brendlé, J., Khouchaf, L., Baron, J., Le Dred, R., & Tuilier, M. H. (1997). Zr-exchanged and pillared beidellite: preparation and characterization by chemical analysis, XRD and Zr K EXAFS. Microporous Materials, 11, 171–183.
Miura, N., Sato, T., Anggraini, S. A., Ikeda, H., & Zhuiykov, S. (2014). A review of mixed-potential type zirconia-based gas sensors. Ionics, 20, 901–925.
Mnasri, S., & Frini-Srasra, N. (2013). Synthesis, characterization and catalytic evaluation of zirconia-pillared bentonite for 1, 3-dioxalane synthesis. Surface Engineering and Applied Electrochemistry, 49, 336–347.
Nagarajan, V., & Rao, K. (1990). Thermally induced chemical and structural changes in alumina-zirconia-silica gels during the formation of ceramic composites. Journal of Solid State Chemistry, 88, 419–428.
Ngnie, G., Dedzo, G. K., & Detellier, C. (2016). Synthesis and catalytic application of palladium nanoparticles supported on kaolinite-based nanohybrid materials. Dalton transactions, 45, 9065–9072.
Ohtsuka, K., Hayashi, Y., & Suda, M. (1993). Microporous zirconia-pillared clays derived from three kinds of zirconium polynuclear ionic species. Chemistry of Materials, 5, 1823–1829.
Olejnik, S., Aylmore, L. A. G., Posner, A. M., & Quirk, J. P. (1968). Infrared spectra of kaolin mineral-dimethyl sulfoxide complexes. The Journal of Physical Chemistry, 72, 241–249.
Sari, A., & Tuzen, M. (2014). Cd(II) adsorption from aqueous solution by raw and modified kaolinite. Applied Clay Science, 88–89, 63–72.
Shirsath, S. R., Patil, A. P., Patil, R., Naik, J. B., Gogate, P. R., & Sonawane, S. H. (2013). Removal of Brilliant Green from wastewater using conventional and ultrasonically prepared poly(acrylic acid) hydrogel loaded with kaolin clay: A comparative study. Ultrasonics Sonochemistry, 20, 914–923.
Singh, B. & Mackinnon, I. D. (1999). Intercalation of kaolins by alkaline earth metal salts. Pp. 489–495 in 11th International Clay Conference, Ottawa.
Solovkin, A. S., & Tsvetkova, Z. N. (1962). The chemistry of aqueous solutions of zirconium salts (Does the zirconyl ion exist?). Russian Chemical Reviews, 31, 655–699.
Štefanić, G., Musić, S., Popović, S., & Furić, K. (1996). Formation of ZrO2 by the thermal decomposition of zirconium salts. Croatica Chemica Acta,69, 223–239.
Swindale, L. D. (1975). The crystallography of minerals of the kaolin group. Pp. 121–154 in Soil Components: Vol. 2: Inorganic Components (J. E. Gieseking, editor). Springer, Berlin Heidelberg.
Takagi, S. (1954). Zirconium compounds. I. Thermal decomposition of zirconium chloride octahydrate. Journal of the Chemical Society of Japan, 75, 637–639.
Tunney, J. J., & Detellier, C. (1993). Interlamellar covalent grafting of organic units on kaolinite. Chemistry of Materials, 5, 747–748.
Valášková, M., Tokarský, J., Hundáková, M., Zdrálková, J., & Smetana, B. (2013). Role of vermiculite and zirconium–vermiculite on the formation of zircon–cordierite nanocomposites. Applied Clay Science, 75, 100–108.
Valverde, J. L., de Lucas, A., Sánchez, P., Dorado, F., & Romero, A. (2003). Cation exchanged and impregnated Ti-pillared clays for selective catalytic reduction of NOx by propylene. Applied Catalysis B: Environmental, 43, 43–56.
Vaughan, D. E. (1994). Pillared interlayered kandite clay compositions. Google Patents.
Vera, C. R., Pieck, C. L., Shimizu, K., & Parera, J. M. (2002). Tetragonal structure, anionic vacancies and catalytic activity of SO42−-ZrO2 catalysts for n-butane isomerization. Applied Catalysis A: General, 230, 137–151.
Wada, K. (1961). Lattice expansion of kaolin minerals by treatment with potassium acetate. American Mineralogist: Journal of Earth and Planetary Materials, 46, 78–91.
Wang, X., Liu, T., Yu, J., Li, L., & Zhang, X. (2019). A new application of CexZr1–xO2 as dense diffusion barrier in limiting current oxygen sensor. Sensors and Actuators B: Chemical, 285, 391–397.
Zaharia, A., Perrin, F.-X., Teodorescu, M., Radu, A.-L., Iordache, T.-V., Florea, A.-M., Donescu, D., & Sarbu, A. (2015). New organophilic kaolin clays based on single-point grafted 3-aminopropyl dimethylethoxysilane. Physical Chemistry Chemical Physics, 17, 24908–24916.
Zhang, Q.-h., Chen, G.-q., & Xing, T.-l. (2017). Silk flame retardant finish by ternary silica sol containing boron and nitrogen. Applied Surface Science, 421, 52–60.
ACKNOWLEDGMENTS
The authors are grateful to the Editor in Chief and the anonymous reviewers for their constructive criticism and valuable comments which helped to improve the manuscript. The authors are also grateful to the National Research Centre, Egypt, for partial financial support of this study via project No AR110903.
Funding
Funding sources are as stated in the Acknowledgments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Supplementary Information
ESM 1
(DOCX 14804 kb)
Rights and permissions
About this article
Cite this article
Abou-El-Sherbini, K.S., Wahba, M.A., Drweesh, E.A. et al. ZIRCONIA-INTERCALATED KAOLINITE: SYNTHESIS, CHARACTERIZATION, AND EVALUATION OF METAL-ION REMOVAL ACTIVITY. Clays Clay Miner. 69, 463–476 (2021). https://doi.org/10.1007/s42860-021-00134-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-021-00134-9