Abstract
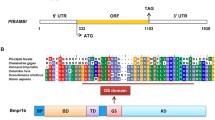
Bone morphogenetic proteins (BMPs), which are members of the superfamily of transforming growth factor-β (TGF-β), are known both in vitro and in vivo for their osteoinduction properties on the osteoblastic cells. Its role in the mollusk shell formation has also been gradually established. Using Haliotis diversicolor as a model, we characterized the HdBMP2/4 gene in the mantle tissue and showed its expression in the outer fold epithelium (particularly at the periostracal groove) the epithelial site which is involved in shell formation, both prismatic and nacreous layers. Shell notching experiments following gene analysis by qPCR revealed the upregulation of the HdBMP2/4 gene up to 3.2-fold than that of the control animals. In vitro treatments of the preosteoblastic cells, MC3T3-E1 with HdBMP2/4 synthetic peptide demonstrated the enhanced effect of many osteogenic genes that are known to regulate bone and shell biomineralization including ALP, Runx2, and OCN with 2–4 fold-change throughout 14 days of culture. In addition, the increased deposition of calcium-based mineral (as assessed by Alizarin red staining) of the treated cells was comparable to the ascorbic acid (Vit C) + glycerophosphate positive control which revealed the enhanced effect of HdBMP2/4 peptide on matrix biomineralization of the preosteoblastic cells. In conclusion, these results indicated the presence of the HdBMP2/4 gene in the mantle tissue at the site involved in shell formation and the effect of the HdBMP2/4 knuckle epitope peptide in osteoinduction in vitro.






Similar content being viewed by others
References
Abula K, Muneta T, Miyatake K, Yamada J, Matsukura Y, Inoue M (2015) Elimination of BMP7 from the developing limb mesenchyme leads to articular cartilage degeneration and synovial inflammation with increased age. FEBS Lett 589:1240–1248
Atlan G, Delattre O, Berland S, LeFaou A, Nabias G, Cot D (1999) The interface between bone and nacre implants in sheep. Biomater 20:1017–1022
Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L (2013) BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng 6:32–52
Berland S, Delattre O, Borzeix S, Catonne Y, Lopez E (2005) Nacre/bone interface changes in durable nacre endosseous implants in sheep. Biomater 26:2767–2773
Camprasse G, Camprasse S, Gill GA (1998) Substitution of the dental root by aquatic invertebrate skeletons in animals and man. C R Acad Sci III Sci Vie 307:485–491
Camprasse S, Camprasse G, Pouzol M, Lopez E (1990) Artificial dental root made of natural calcium carbonate (Bioracin). Clin Mater 5:235–250
Carmel MD, Kathryn G, Daniel JJ, Bernard MD (2011) Ultrastructure of the Mantle of the Gastropod Haliotis asinina and Mechanisms of Shell Regionalization. Cells Tissues Organs 194:103–107
Chaturvedi R, Singha PK, Dey S (2013) Water Soluble Bioactives of Nacre Mediate Antioxidant Activity and Osteoblast Differentiation. PLoS One 8:e84584
Checa A (2000) A new model for periostracum and shell formation in Unionidae (Bivalvia, Mollusca). Tissue Cell 32:405–416
Chen G, Deng C, Li YP (2012) TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8:272–288
Cheng Y, Zhang W, Fan H, Xu P (2018) Water-soluble nano-pearl powder promotes MC3T3-E1 cell differentiation by enhancing autophagy via the MEK/ERK signaling pathway. Mol Med Rep 18:993–1000
David WG, Hyuk-Jae K, Han-Sung J (2015) Osteogenic potency of nacre on human mesenchymal stem cells. Mol Cells 38:267–272
Derynck R, Jarrett JA, Chen EY, Goeddel DV (1986) The murine transforming growth factor-beta precursor. J Biol Chem 261:4377–4379
Dubois CM, Blanchette F, Laprise MH, Leduc R, Grondin F, Seidah NG (2001) Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme. Am J Pathol 158:305–316
Fleury C, Marie B, Luquet G, Josse C, Serpentini A, Lebel A (2008) Shell repair process in the green ormer Haliotis tuberculata: A histological and microstructural study. Tissue Cell 40:207–218
Hayashi M, Maeda S, Aburatani H, Kitamura K, Miyoshi H, Miyazono K, Imamura T (2008) Pitx2 prevents osteoblastic transdifferentiation of myoblasts by bone morphogenetic proteins. J Biol Chem 283:565–571
Kanakaris NK, Giannoudis PV (2008) Clinical applications of bone morphogenetic proteins: current evidence. J Surg Orthop Adv 17:133–146
Kocot KM, Aguilera F, McDougall CM, Jackson DJ, Degnan BM (2016) Seashell diversity and rapidly evolving secretomes: insights into the evolution of biomineralization. Front Zool 13:33
Kirsch T, Nickel J, Sebald W (2000) BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J 19:3314–3324
Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG, Hwang YS (2005) Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J Biol Chem 280:35579–35587
Lian JB, Stein GS, Javed A, Wijnen AJ, Stein JL, Montecino M, Hassan MQ (2006) Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7:1–16
Lian JB, Stein GS, Stein JL, van Wijnen AJ (1998) Osteocalcin gene promoter: unlocking the secrets for the regulation of osteoblast growth and differentiation. J Cell Biochem Suppl 30–31:62–72
Liu HL, Liu SF, Ge YJ, Liu J, Wang XY, Xie LP, Zhang RQ (2007) Identification and characterization of a biomineralization related gene PFMG1 highly expressed in the mantle of Pinctada fucata. Biochem 46:844–851
Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL (2007) Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 25:665–677
Ma JY, Wong KL, Xu ZY, Au AY, Lee NL, Su C (2016) N16, a nacreous protein, inhibits osteoclast differentiation and enhances osteogenesis. J Nat Prod 79:204–212
Mann K, Cerveau N, Gummich M, Fritz M, Mann M, Jackson DJ (2018) In-depth proteomic analyses of Haliotis laevigata (greenlip abalone) nacre and prismatic organic shell matrix. Proteome Sci 16:1–25
Marin F, Roy NL, Marie B (2012) The formation and mineralization of the mollusk shell. Front Biosci S4:1099–1125
Matsushiro A, Miyashita T (2004) Evolution of hard-tissue mineralization: comparison of the inner skeletal system and the outer shell system. J Bone Miner Metab 22:163–169
Miyamoto H, Miyashita T, Okushima M, Nakano S, Morita T, Matsushiro A (1996) A carbonic anhydrase from the nacreous layer in oyster pearls. Proc Natl Acad Sci 93:9657–9660
Miyashita T, Hanashita T, Toriyama M, Takagi R, Akashika T, Higashikubo N (2008) Gene cloning and biochemical characterization of the BMP-2 of Pinctada fucata. Biosci Biotechnol Biochem 72:37–47
Nelsen SM, Christian JL (2009) Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem 284:27157–27166
Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T (2012) Regulation of bone and cartilage development by the network between BMP signaling and transcription factors. e-J Biochem 151:247–254
Park SY, Kim KH, Kim S, Lee YM, Seol YJ (2019) BMP-s gene delivery-based bone regeneration in dentistry. Pharmaceutics 11:393
Pascaretti GF, Libouban H, Camprasse G, Camprasse S, Mallet R, Chappard D (2014) The interface between nacre and bone after implantation in the sheep: A nanotomographic and Raman study: Bone-nacre interface. J Raman Spectroc 45:558–564
Peverali FA, Basdra EK, Papavassiliou AG (2001) Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. Mol Med 7:68–78
Ray C, Combes C, Drouet C, Glimcher MJ (2009) Bone mineral: update on chemical composition and structure. Osteoporosis Int 20:1013–1021
Samata T, Hayashi N, Kono M, Hasegawa K, Horita C, Akera S (1999) A new matrix protein family is related to the nacreous layer formation of Pinctada fucata. FEBS Lett 462:225–229
Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF (1992) Recombinant human osteogenic protein-1 (HOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem 267:20352–20362
Saito A, Suzuki Y, Ogata SI, Ohtsuki C, Tanihara M (2003) Activation of osteoprogenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochem Biophys Acta 1651:60–67
Sharapova NE, Kotnova AP, Galushkina ZM, Lavrova NV, Poletaeva NN, Tukhvatulin AE (2010) Production of the recombinant human bone morphogenetic protein 2 in escherichia coli and testing of its biological activity in vitro and in vivo. Mol Biol 44:923–930
Shen Q, Christakos S (2005) The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem 280:40589–40598
Sroga GE, Vashishth D (2012) Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10:141–150
Sudo S, Fujikawa T, Nagakura T, Ohkubo T, Sakaguchi K, Tanaka M (1997) Structures of mollusk shell framework proteins. Nature 387:563–564
Takagi R, Miyashita T (2010) Prismin: a new matrix protein family in the Japanese pearl oyster (Pinctada fucata) involved in prismatic layer formation. Zoolog Sci 27:416–426
Ulsamer A, Ortuno MJ, Ruiz S, Susperregui ARG, Osses N, Rosa JL (2008) BMP-2 induces Osterix expression through up-regulation of Dlx5 and its phosphorylation by p38. J Biol Chem 283:3816–3826
Wang X, Harimoto K, Fuji R, Liu J, Li L, Wang P (2015) Pinctada fucata mantle gene 4 (PFMG4) from pearl oyster mantle enhances osteoblast differentiation. Biosci Biotech Bioch 79:558–565
Weiss I, Kaufmann S, Mann K, Fritz M (2000) Purification and Characterization of Perlucin and Perlustrin, Two New Proteins from the Shell of the Mollusc Haliotis laevigata. Biochem Biophys Res Commun 267:17–21
Westbroek P, Marin F (1998) A marriage of bone and nacre. Nature 392:861–862
Yano M, Nagai K, Morimoto K, Miyamoto H (2007) A novel nacre protein N19 in the pearl oyster Pinctada fucata. Biochem Biophys Res Commun 362:158–163
Zaidi SK, Javed A, Choi JY, Wijnen AJ, Stein JL, Lian JB (2001) A specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to the transactivation of the osteocalcin gene. J Cell Sci 114:3093–3102
Zhao M, Shi Y, He M, Huang WQ (2016) PfSMAD4 plays a role in biomineralization and can transduce bone morphogenetic protein-s signals in the pearl oyster Pinctada fucata. BMC Dev Biol 16:9
Zhang C, Li S, Ma Z, Xie L, Zhang R (2006) A Novel Matrix Protein p10 from the Nacre of Pearl Oyster (Pinctada fucata) and Its Effects on Both CaCO3 Crystal Formation and Mineralogenic Cells. Mar Biotechnol 8:624–633
Zhang G, Brion A, Willemin AS, Piet MH, Moby V, Bianchi A (2017) Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J Biomed Mater Res Part A 105:662–672
Zhang Y, Xie L, Meng Q, Jing T, Pu R, Chen L (2003) A novel matrix protein participating in the nacre framework formation of pearl oyster, Pinctada fucata. Comp Biochem Physiol B 135:565–573
Zhang Y, Ma Y, Yang M, Min S, Yao J, Liangjun ZL (2011) Expression, purification, and refolding of a recombinant human bone morphogenetic protein 2 in vitro. Protein Exp Purif 75:155–160
Zoccola D, Moya A, Beranger GE (2009) Specific expression of BMP2/4 ortholog in biomineralizing tissue of corals and action on mouse BMP receptor. Mar Biotechnol 11:260
Acknowledgements
We thank the Center of Nanoimaging (CNI) and the Central Instrument Facility (CIF), Faculty of Science, Mahidol University, for providing instrumental support throughout this work.
Funding
This research project is supported by Mahidol University (to SA) and Naresuan University (to CS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study was carried out in accordance with the protocol approved by the Animal Care Committee, Faculty of Science, Mahidol University (Protocol # MUSC-60–040-390).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suwannasing, C., Buddawong, A., Khumpune, S. et al. Bone Morphogenetic Protein 2/4 in Mollusk, Haliotis diversicolor: Its Expression and Osteoinductive Function In Vitro. Mar Biotechnol 23, 836–846 (2021). https://doi.org/10.1007/s10126-021-10071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-021-10071-2