Abstract
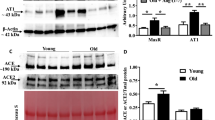
Our past study showed that coronary arterioles isolated from adipose-derived stromal vascular fraction (SVF)–treated rats showed amelioration of the age-related decrease in vasodilation to beta-adrenergic receptor (β-AR) agonist and improved β-AR-dependent coronary flow and microvascular function in a model of advanced age. We hypothesized that intravenously (i.v.) injected SVF improves coronary microvascular function in aged rats by re-establishing the equilibrium of the negative regulators of the internal adrenergic signaling cascade, G-protein receptor kinase 2 (GRK2) and G-alpha inhibitory (Gαi) proteins, back to youthful levels. Female Fischer-344 rats aged young (3 months, n = 24), old (24 months, n = 26), and old animals that received 1 × 107 green fluorescent protein (GFP+) SVF cells (O + SVF, n = 11) 4 weeks prior to sacrifice were utilized. Overnight urine was collected prior to sacrifice for catecholamine measurements. Cardiac samples were used for western blotting while coronary arterioles were isolated for pressure myography studies, immunofluorescence staining, and RNA sequencing. Coronary microvascular levels of the β1 adrenergic receptor are decreased with advancing age, but this decreased expression was rescued by SVF treatment. Aging led to a decrease in phosphorylated GRK2 in cardiomyocytes vs. young control with restoration of phosphorylation status by SVF. In vessels, there was no change in genetic transcription (RNAseq) or protein expression (immunofluorescence); however, inhibition of GRK2 (paroxetine) led to improved vasodilation to norepinephrine in the old control (OC) and O + SVF, indicating greater GRK2 functional inhibition of β1-AR in aging. SVF works to improve adrenergic-mediated vasodilation by restoring the β1-AR population and mitigating signal cascade inhibitors to improve vasodilation.








Similar content being viewed by others
Abbreviations
- BF:
-
blood flow
- HF:
-
heart failure
- β-AR:
-
beta-adrenergic receptor
- α-AR:
-
alpha-adrenergic receptor
- AC:
-
adenylyl cyclase
- Gαi:
-
alpha subunit of inhibitory G-protein
- Gs:
-
stimulatory G-protein
- GRK:
-
G-protein receptor kinase
- i.v.:
-
intravenous
- SVF:
-
adipose-derived stromal vascular fractions
- CFR:
-
coronary flow reserve
- YC:
-
young control
- OC:
-
old control
- GFP+:
-
transgenic green fluorescent protein positive
- O + SVF:
-
old treated with adipose-derived stromal vascular fractions
- LAD:
-
left anterior descending coronary artery
- PSS:
-
physiological salt solution
- NE:
-
norepinephrine
- Parox:
-
paroxetine HCl
- NaF:
-
sodium fluoride
- CPG:
-
CPG20712A
- Dob:
-
dobutamine
- ICI:
-
ICI118551
- SNP:
-
sodium nitroprusside
- RT:
-
room temperature
- ROI:
-
region of interest
- LV:
-
left ventricular
- BW:
-
body weight (grams)
- Epi:
-
epinephrine
- 5HT:
-
serotonin
- DA:
-
dopamine
- 5-HIAA:
-
metabolite of serotonin
- Dmax:
-
maximum diameter (μm)
- P-GRK2:
-
phosphorylated G-protein receptor kinase 2
- Gnαo1:
-
G-protein subunit alpha-o1
- Gnαi2:
-
G-protein subunit alpha-i2
- Sgsm2:
-
small G-protein signaling modulator-2
- AC6:
-
adenylyl cyclase isoform 6
- CVD:
-
cardiovascular disease
- CMD:
-
coronary microvascular disease
- MI:
-
myocardial ischemia
- MSC:
-
mesenchymal stem cell
- NO:
-
nitric oxide
References
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110(13):1799–805.
Tracy E, Rowe G, LeBlanc AJ. Cardiac tissue remodeling in healthy aging: the road to pathology. Am J Phys Cell Phys. 2020;319(1):C166–C82. https://doi.org/10.1152/ajpcell.00021.2020 Epub 2020/05/21. PubMed PMID: 32432929; PubMed Central PMCID: PMCPMC7468888.
Ziegler MG, Lake CR, Kopin IJ. Plasma noradrenaline increases with age. Nature. 1976;261(5558):333–5. Epub 1976/05/27. https://doi.org/10.1038/261333a0.
Xiao RP, Lakatta EG. Deterioration of beta-adrenergic modulation of cardiovascular function with aging. Ann N Y Acad Sci. 1992;673:293–310 Epub 1992/12/26.
White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, et al. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation. 1994;90(3):1225–38 Epub 1994/09/01.
Pan HY, Hoffman BB, Pershe RA, Blaschke TF. Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986;239(3):802–7 Epub 1986/12/01.
Mendzef SD, Slovinski JR. Neurohormones and heart failure. Nurs Clin North Am. 2004;39(4):845–61. Epub 2004/11/25. https://doi.org/10.1016/j.cnur.2004.07.004.
Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415(6868):206–12. Epub 2002/01/24. https://doi.org/10.1038/415206a.
Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol. 1997;80(11A):26L–40L. Epub 1997/12/31. https://doi.org/10.1016/s0002-9149(97)00846-1.
Satwani S, Dec GW, Narula J. Beta-adrenergic blockers in heart failure: review of mechanisms of action and clinical outcomes. J Cardiovasc Pharmacol Ther. 2004;9(4):243–55. Epub 2005/01/29. https://doi.org/10.1177/107424840400900404.
Velasco A, Solow E, Price A, Wang Z, Arbique D, Arbique G, et al. Differential effects of nebivolol vs. metoprolol on microvascular function in hypertensive humans. Am J Physiol Heart Circ Physiol. 2016;311(1):H118–24. https://doi.org/10.1152/ajpheart.00237.2016 Epub 2016/05/21. PubMed PMID: 27199121; PubMed Central PMCID: PMCPMC4967201.
Rowe G, Kelm NQ, Beare JE, Tracy E, Yuan F, LeBlanc AJ. Enhanced beta-1 adrenergic receptor responsiveness in coronary arterioles following intravenous stromal vascular fraction therapy in aged rats. Aging (Albany NY). 2019;11(13):4561–78. https://doi.org/10.18632/aging.102069 Epub 2019/07/13. PubMed PMID: 31296794; PubMed Central PMCID: PMCPMC6660031.
Brodde OE, Michel MC, Zerkowski HR. Signal transduction mechanisms controlling cardiac contractility and their alterations in chronic heart failure. Cardiovasc Res. 1995;30(4):570–84 Epub 1995/10/01.
Neumann J, Schmitz W, Scholz H, von Meyerinck L, Doring V, Kalmar P. Increase in myocardial Gi-proteins in heart failure. Lancet. 1988;2(8617):936–7. Epub 1988/10/22. https://doi.org/10.1016/s0140-6736(88)92601-3.
Feldman AM, Cates AE, Veazey WB, Hershberger RE, Bristow MR, Baughman KL, et al. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest. 1988;82(1):189–97. https://doi.org/10.1172/JCI113569 Epub 1988/07/01. PubMed PMID: 2839545; PubMed Central PMCID: PMCPMC303493.
Bohm M, Gierschik P, Knorr A, Schmidt U, Weismann K, Erdmann E. Cardiac adenylyl cyclase, beta-adrenergic receptors, and G proteins in salt-sensitive hypertension. Hypertension. 1993;22(5):715–27. Epub 1993/11/01. https://doi.org/10.1161/01.hyp.22.5.715.
Ferrara N, Bohm M, Zolk O, O'Gara P, Harding SE. The role of Gi-proteins and beta-adrenoceptors in the age-related decline of contraction in guinea-pig ventricular myocytes. J Mol Cell Cardiol. 1997;29(2):439–48 Epub 1997/02/01.
Narayanan N, Tucker L. Autonomic interactions in the aging heart: age-associated decrease in muscarinic cholinergic receptor mediated inhibition of beta-adrenergic activation of adenylate cyclase. Mech Ageing Dev. 1986;34(3):249–59 Epub 1986/05/01.
Lamba S, Abraham WT. Alterations in adrenergic receptor signaling in heart failure. Heart Fail Rev. 2000;5(1):7–16. Epub 2005/10/18. https://doi.org/10.1023/A:1009885822076.
Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25(5):1154–61. Epub 1995/04/01. https://doi.org/10.1016/0735-1097(94)00543-y.
Schutzer WE, Xue H, Reed J, Oyama T, Beard DR, Anderson S, et al. Age-related beta-adrenergic receptor-mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vasc Pharmacol. 2011;55(5-6):178–88. Epub 2011/09/29. https://doi.org/10.1016/j.vph.2011.09.001.
El-Armouche A, Zolk O, Rau T, Eschenhagen T. Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res. 2003;60(3):478–87. Epub 2003/12/09. https://doi.org/10.1016/j.cardiores.2003.09.014.
Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87(2):454–63. Epub 1993/02/01. https://doi.org/10.1161/01.cir.87.2.454.
Johnson MD, Zhou Y, Friedman E, Roberts J. Expression of G protein alpha subunits in the aging cardiovascular system. J Gerontol A Biol Sci Med Sci. 1995;50A(1):B14–9. Epub 1995/01/01. https://doi.org/10.1093/gerona/50a.1.b14.
Nash CA, Nelson CP, Mistry R, Moeller-Olsen C, Christofidou E, Challiss RAJ, et al. Differential regulation of beta2-adrenoceptor and adenosine A2B receptor signalling by GRK and arrestin proteins in arterial smooth muscle. Cell Signal. 2018;51:86–98. Epub 2018/08/04. https://doi.org/10.1016/j.cellsig.2018.07.013.
Kelm NQ, Beare JE, Yuan F, George M, Shofner CM, Keller BB, et al. Adipose-derived cells improve left ventricular diastolic function and increase microvascular perfusion in advanced age. PLoS One. 2018;13(8):e0202934. Epub 2018/08/25. https://doi.org/10.1371/journal.pone.0202934.
Council NR. Guide for the care and use of laboratory animals: eighth edition. Washington: The National Academies Press; 2011. 246 p.
Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–501. Epub 2000/01/05. https://doi.org/10.1093/gerona/54.11.b492.
Leblanc AJ, Touroo JS, Hoying JB, Williams SK. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302(4):H973–82. Epub 2011/12/06. https://doi.org/10.1152/ajpheart.00735.2011.
Xie Z, Lorkiewicz P, Riggs DW, Bhatnagar A, Srivastava S. Comprehensive, robust, and sensitive UPLC-MS/MS analysis of free biogenic monoamines and their metabolites in urine. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1099:83–91. https://doi.org/10.1016/j.jchromb.2018.09.012 Epub 2018/09/25. PubMed PMID: 30248561; PubMed Central PMCID: PMCPMC6398444.
Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Phys. 1986;251(4 Pt 2):H779–88.
Mboge MY, Chen Z, Khokhar D, Wolff A, Ai L, Heldermon CD, et al. A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochem J. 2019;476(10):1497–513. Epub 2019/05/11. https://doi.org/10.1042/BCJ20190177.
Lanceta L, O'Neill C, Lypova N, Li X, Rouchka E, Waigel S, et al. Transcriptomic profiling identifies differentially expressed genes in palbociclib-resistant ER+ MCF7 breast cancer cells. Genes (Basel). 2020;11(4):467. https://doi.org/10.3390/genes11040467 Epub 2020/04/30. PubMed PMID: 32344635; PubMed Central PMCID: PMCPMC7230561.
Miralda I, Vashishta A, Rogers MN, Rouchka EC, Li X, Waigel S, et al. Whole transcriptome analysis reveals that Filifactor alocis modulates TNFalpha-stimulated MAPK activation in human neutrophils. Front Immunol. 2020;11:497. https://doi.org/10.3389/fimmu.2020.00497 Epub 2020/05/07. PubMed PMID: 32373107; PubMed Central PMCID: PMCPMC7179764.
Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8(2):210–20. https://doi.org/10.1016/j.jcmg.2014.12.008 Epub 2015/02/14. PubMed PMID: 25677893; PubMed Central PMCID: PMCPMC4384521.
Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–6. Epub 1994/08/01. https://doi.org/10.1016/0735-1097(94)90305-0.
LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, et al. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Phys Regul Integr Comp Phys. 2009;297(6):R1713–23. https://doi.org/10.1152/ajpregu.00178.2009 Epub 2009/10/09. PubMed PMID: 19812360; PubMed Central PMCID: PMCPMC2803626.
Beckman JA, Duncan MS, Damrauer SM, Wells QS, Barnett JV, Wasserman DH, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140(6):449–58. https://doi.org/10.1161/CIRCULATIONAHA.119.040672 Epub 2019/07/10. PubMed PMID: 31280589; PubMed Central PMCID: PMCPMC6682431.
Nguyen PK, Rhee JW, Wu JC. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 2016;1(7):831–41. https://doi.org/10.1001/jamacardio.2016.2225 Epub 2016/10/21. PubMed PMID: 27557438; PubMed Central PMCID: PMCPMC5349705.
Dubey NK, Mishra VK, Dubey R, Deng YH, Tsai FC, Deng WP. Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. Int J Mol Sci. 2018;19(8):2200. https://doi.org/10.3390/ijms19082200 Epub 2018/08/01. PubMed PMID: 30060511; PubMed Central PMCID: PMCPMC6121360.
Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 2020;9(9):985–1006. https://doi.org/10.1002/sctm.19-0446 Epub 2020/06/05. PubMed PMID: 32497410; PubMed Central PMCID: PMCPMC7445024.
Zhu M, Lu F, Gao J, Liao Y. Effects of different human adipose-derived cells in promoting human adipose tissue engraftment in nude mice. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32(9):1265–9 Epub 2012/09/19.
Kore RA, Wang X, Ding Z, Griffin RJ, Tackett AJ, Mehta JL. MSC exosome-mediated cardioprotection in ischemic mouse heart comparative proteomics of infarct and peri-infarct areas. Mol Cell Biochem. 2021;476(4):1691–704 Epub 2021/01/11. doi: 10.1007/s11010-020-04029-6. PubMed PMID: 33423165; PubMed Central PMCID: PMCPMC8186026.
Cheng L, Zhang K, Wu S, Cui M, Xu T. Focus on mesenchymal stem cell-derived exosomes: opportunities and challenges in cell-free therapy. Stem Cells Int. 2017;2017:6305295. https://doi.org/10.1155/2017/6305295 Epub 2018/02/08. PubMed PMID: 29410682; PubMed Central PMCID: PMCPMC5749272.
Paul A, Srivastava S, Chen G, Shum-Tim D, Prakash S. Functional assessment of adipose stem cells for xenotransplantation using myocardial infarction immunocompetent models: comparison with bone marrow stem cells. Cell Biochem Biophys. 2013;67(2):263–73. Epub 2011/12/30. https://doi.org/10.1007/s12013-011-9323-0.
Rasmussen JG, Frobert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23(2):195–206. Epub 2012/12/06. https://doi.org/10.3727/096368912X659871.
Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20(6):867–76. Epub 2007/11/06. https://doi.org/10.1159/000110447.
Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014;11(1):1. https://doi.org/10.1186/1476-9255-11-1 Epub 2014/01/09. PubMed PMID: 24397734; PubMed Central PMCID: PMCPMC3895743.
Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. 2017;8(1):145. https://doi.org/10.1186/s13287-017-0598-y Epub 2017/06/18. PubMed PMID: 28619097; PubMed Central PMCID: PMCPMC5472998.
Wilson A, Hodgson-Garms M, Frith JE, Genever P. Multiplicity of mesenchymal stromal cells: finding the right route to therapy. Front Immunol. 2019;10:1112. https://doi.org/10.3389/fimmu.2019.01112 Epub 2019/06/06. PubMed PMID: 31164890; PubMed Central PMCID: PMCPMC6535495.
Stivers KB, Beare JE, Chilton PM, Williams SK, Kaufman CL, Hoying JB. Adipose-derived cellular therapies in solid organ and vascularized-composite allotransplantation. Curr Opin Organ Transplant. 2017;22(5):490–8. Epub 2017/09/06. https://doi.org/10.1097/MOT.0000000000000452.
Engelhardt S, Bohm M, Erdmann E, Lohse MJ. Analysis of beta-adrenergic receptor mRNA levels in human ventricular biopsy specimens by quantitative polymerase chain reactions: progressive reduction of beta 1-adrenergic receptor mRNA in heart failure. J Am Coll Cardiol. 1996;27(1):146–54. Epub 1996/01/01. https://doi.org/10.1016/0735-1097(95)00425-4.
Tsujimoto G, Hoffman BB. Desensitization of beta-adrenergic receptor-mediated vascular smooth muscle relaxation. Mol Pharmacol. 1985;27(2):210–7 Epub 1985/02/01.
Parmley WW. Pathophysiology of congestive heart failure. Am J Cardiol. 1985;56(2):7A–11A. Epub 1985/07/10. https://doi.org/10.1016/0002-9149(85)91199-3.
Ferro A, Kaumann AJ, Brown MJ. Beta-adrenoceptor subtypes in human coronary artery: desensitization of beta 2-adrenergic vasorelaxation by chronic beta 1-adrenergic stimulation in vitro. J Cardiovasc Pharmacol. 1995;25(1):134–41 Epub 1995/01/01.
Penela P, Ribas C, Mayor F Jr. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15(11):973–81. Epub 2003/09/23. https://doi.org/10.1016/s0898-6568(03)00099-8.
Liang W, Curran PK, Hoang Q, Moreland RT, Fishman PH. Differences in endosomal targeting of human (beta)1- and (beta)2-adrenergic receptors following clathrin-mediated endocytosis. J Cell Sci. 2004;117(Pt 5):723–34. Epub 2004/01/22. https://doi.org/10.1242/jcs.00878.
Schutzer WE, Reed JF, Bliziotes M, Mader SL. Upregulation of G protein-linked receptor kinases with advancing age in rat aorta. Am J Phys Regul Integr Comp Phys. 2001;280(3):R897–903. Epub 2001/02/15. https://doi.org/10.1152/ajpregu.2001.280.3.R897.
Shiina T, Kawasaki A, Nagao T, Kurose H. Interaction with beta-arrestin determines the difference in internalization behavor between beta1- and beta2-adrenergic receptors. J Biol Chem. 2000;275(37):29082–90. Epub 2000/06/23. https://doi.org/10.1074/jbc.M909757199.
Noor N, Patel CB, Rockman HA. Beta-arrestin: a signaling molecule and potential therapeutic target for heart failure. J Mol Cell Cardiol. 2011;51(4):534–41. https://doi.org/10.1016/j.yjmcc.2010.11.005 Epub 2010/11/16. PubMed PMID: 21074538; PubMed Central PMCID: PMCPMC3063861.
Rengo G, Lymperopoulos A, Leosco D, Koch WJ. GRK2 as a novel gene therapy target in heart failure. J Mol Cell Cardiol. 2011;50(5):785–92. https://doi.org/10.1016/j.yjmcc.2010.08.014 Epub 2010/08/31. PubMed PMID: 20800067; PubMed Central PMCID: PMCPMC3005526.
Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7(277):277ra31. https://doi.org/10.1126/scitranslmed.aaa0154 Epub 2015/03/06. PubMed PMID: 25739765; PubMed Central PMCID: PMCPMC4768806.
Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, et al. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. 2012;7(11):1830–9. https://doi.org/10.1021/cb3003013 Epub 2012/08/14. PubMed PMID: 22882301; PubMed Central PMCID: PMCPMC3500392.
Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61(4):749–58. Epub 2002/03/20. https://doi.org/10.1124/mol.61.4.749.
Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(Suppl 2):1–15. Epub 1984/01/01. https://doi.org/10.2165/00003495-198400282-00002.
Fleming JW, Wisler PL, Watanabe AM. Signal transduction by G proteins in cardiac tissues. Circulation. 1992;85(2):420–33. Epub 1992/02/01. https://doi.org/10.1161/01.cir.85.2.420.
Weber LP, Chow WL, Abebe W, MacLeod KM. Enhanced contractile responses of arteries from streptozotocin diabetic rats to sodium fluoride. Br J Pharmacol. 1996;118(1):115–22. https://doi.org/10.1111/j.1476-5381.1996.tb15373.x Epub 1996/05/01. PubMed PMID: 8733583; PubMed Central PMCID: PMCPMC1909482.
Cushing DJ, Sabouni MH, Brown GL, Mustafa SJ. Fluoride produces endothelium-dependent relaxation and endothelium-independent contraction in coronary artery. J Pharmacol Exp Ther. 1990;254(1):28–32 Epub 1990/07/01.
Hippe HJ, Ludde M, Schnoes K, Novakovic A, Lutz S, Katus HA, et al. Competition for Gbetagamma dimers mediates a specific cross-talk between stimulatory and inhibitory G protein alpha subunits of the adenylyl cyclase in cardiomyocytes. Naunyn Schmiedeberg's Arch Pharmacol. 2013;386(6):459–69. Epub 2013/04/26. https://doi.org/10.1007/s00210-013-0876-x.
Navarro G, Cordomi A, Brugarolas M, Moreno E, Aguinaga D, Perez-Benito L, et al. Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain. BMC Biol. 2018;16(1):24. https://doi.org/10.1186/s12915-018-0491-x Epub 2018/03/01. PubMed PMID: 29486745; PubMed Central PMCID: PMCPMC6389107.
Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24(6):765–81. Epub 2003/12/13. https://doi.org/10.1210/er.2000-0026.
Janssen PM, Schillinger W, Donahue JK, Zeitz O, Emami S, Lehnart SE, et al. Intracellular beta-blockade: overexpression of Galpha(i2) depresses the beta-adrenergic response in intact myocardium. Cardiovasc Res. 2002;55(2):300–8. Epub 2002/07/19. https://doi.org/10.1016/s0008-6363(02)00406-6.
Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, Gao E, et al. Gi-biased beta2AR signaling links GRK2 upregulation to heart failure. Circ Res. 2012;110(2):265–74. https://doi.org/10.1161/CIRCRESAHA.111.253260 Epub 2011/12/20. PubMed PMID: 22179058; PubMed Central PMCID: PMCPMC3282829.
Lyons D. Impairment and restoration of nitric oxide-dependent vasodilation in cardiovascular disease. Int J Cardiol. 1997;62(Suppl 2):S101–9. Epub 1998/03/06. https://doi.org/10.1016/s0167-5273(97)00247-7.
Mokhtar SS, Vanhoutte PM, Leung SW, Yusof MI, Wan Sulaiman WA, Mat Saad AZ, et al. Endothelium dependent hyperpolarization-type relaxation compensates for attenuated nitric oxide-mediated responses in subcutaneous arteries of diabetic patients. Nitric Oxide. 2016;53:35–44. Epub 2016/01/16. https://doi.org/10.1016/j.niox.2015.12.007.
Hendriks-Balk MC, Peters SL, Michel MC, Alewijnse AE. Regulation of G protein-coupled receptor signalling: focus on the cardiovascular system and regulator of G protein signalling proteins. Eur J Pharmacol. 2008;585(2-3):278–91. Epub 2008/04/16. https://doi.org/10.1016/j.ejphar.2008.02.088.
Stiles GL, Lefkowitz RJ. Cardiac adrenergic receptors. Annu Rev Med. 1984;35:149–64. Epub 1984/01/01. https://doi.org/10.1146/annurev.me.35.020184.001053.
Ho D, Yan L, Iwatsubo K, Vatner DE, Vatner SF. Modulation of beta-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail Rev. 2010;15(5):495–512. https://doi.org/10.1007/s10741-010-9183-5 Epub 2010/07/27. PubMed PMID: 20658186; PubMed Central PMCID: PMCPMC3655553.
Wu YS, Chen CC, Chien CL, Lai HL, Jiang ST, Chen YC, et al. The type VI adenylyl cyclase protects cardiomyocytes from beta-adrenergic stress by a PKA/STAT3-dependent pathway. J Biomed Sci. 2017;24(1):68. https://doi.org/10.1186/s12929-017-0367-3 Epub 2017/09/06. PubMed PMID: 28870220; PubMed Central PMCID: PMCPMC5584049.
Hachamovitch R, Wicker P, Capasso JM, Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Phys. 1989;256(1 Pt 2):H66–73. Epub 1989/01/01. https://doi.org/10.1152/ajpheart.1989.256.1.H66.
LeBlanc AJ, Uchida S. A step closer to improving cardiac homing of adipose-derived mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2019;316(2):H260–H1. https://doi.org/10.1152/ajpheart.00736.2018 Epub 2018/11/22. PubMed PMID: 30461301; PubMed Central PMCID: PMCPMC6397383.
LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008;295(6):H2280–8. https://doi.org/10.1152/ajpheart.00541.2008 Epub 2008/10/07. PubMed PMID: 18835919; PubMed Central PMCID: PMCPMC2614537.
Acknowledgements
The authors wish to thank the University of Missouri Rat Resource & Research Center (P40OD011062) for the initial GFP+ Fischer-344 breeding pairs, the University of Louisville Bioanalytical Core for urine catecholamine level testing, the University of Louisville Genomics Core for the RNA sequencing, and the University of Louisville Bioinformatics core for the library analysis. The authors also wish to thank Drs. Nolan Boyd and Natia Kelm for their technical expertise and Ms. Michaela Dukes for technical support in western blotting, and Ms. Virginia Steilberg for technical support with isolated coronary arteriole experiments.
Funding
This research was funded by the National Institutes of Health (R01 AG053585), Department of Defense (W81XWH-19-RTRP-IDA and W81XWH-13-2-0057), and the Gheen’s Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All animal surgeries were performed in accordance with protocols approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC-approved protocol #19635) and the NIH Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplemental Fig. 1
Contribution of the β-2 Adrenergic Receptor to Adrenergic-Dependent Vasodilation. Using pressure myography, isolated coronary microvessels from YC, OC, and O+SVF animals were subjected to β-AR agonism with NE with and without the β2-AR inhibitor ICI118551 (a-c) or dobutamine with and without the β2-AR inhibitor ICI118551 (d-f). Data are presented as means±SEM. Significance defined by paired pre- to post-inhibition for analysis using Two-Way Repeated Measures ANOVA with Bonferroni post hoc testing. p≤.05 when pre- vs. post-inhibition (*). (PNG 134 kb)

Supplemental Fig. 2
Contribution of the β-3 Adrenergic Receptor and cAMP to Adrenergic-Dependent Vasodilation. BRL-37344, a β3-AR agonist (a), and 8-Bromo-cAMP, a cAMP donor (b), were added in a dose-dependent manor to isolated coronary microvessels from YC, OC, and O+SVF animals via pressure myography. Data presented as means±SEM. Significance determined as p<.05 when vs. YC (*) and vs. OC (#) utilizing Two-Way Repeated Measures ANOVA with Bonferroni post hoc analysis. (PNG 110 kb)
Supplemental Table 1
RNA transcripts. List of RNA transcripts and expression of differentially expressed genes in isolated coronary microvessels from YC, OC, and O+SVF samples. Significance defined as cuffdiff and DESeq2 for cutoff p<.05. (DOCX 2343 kb)
About this article
Cite this article
Rowe, G., Tracy, E., Beare, J.E. et al. Cell therapy rescues aging-induced beta-1 adrenergic receptor and GRK2 dysfunction in the coronary microcirculation. GeroScience 44, 329–348 (2022). https://doi.org/10.1007/s11357-021-00455-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-021-00455-6