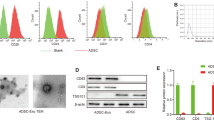
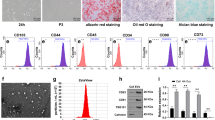
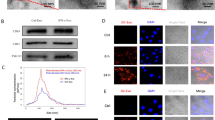
Abstract
Cardiac fibrosis is a difficult clinical puzzle without effective therapy. Exosomes play an important role in alleviating cardiac fibrosis via angiogenesis. This research aimed to assess the effect of bovine milk on cardiac fibrosis. The proangiogenic effect of bovine milk exosomes was analyzed both in isoproterenol (ISO)-induced cardiac fibrosis rats in vivo and in human umbilical vein endothelial cells (HUVECs) after oxygen and glucose deprivation (OGD) in vitro. Results indicated that bovine milk exosomes alleviated the extracellular matrix (ECM) deposition and enhanced the cardiac function in cardiac fibrosis rat. The proangiogenic growth factors were significantly enhanced in rats accepted bovine milk exosomes. Meanwhile, bovine milk exosomes ameliorated the motility, migration, and tube-forming ability of HUVECs after OGD in vitro. Bovine milk exosomes alleviate cardiac fibrosis and enhance cardiac function in cardiac fibrosis rats via enhancing angiogenesis. Bovine milk exosomes may represent a potential strategy for the treatment of cardiac fibrosis.
Graphical abstract





Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- ISO:
-
Isoproterenol
- PBS:
-
Phosphate-buffered saline
- H&E:
-
Hematoxylin and eosin
- LVIDd:
-
Left ventricular internal dimension at end-diastole
- LVIDs:
-
Left ventricular internal dimension at end-systole
- LVPWs:
-
Left ventricular posterior wall thickness at end-systole
- LVPWd:
-
Left ventricular posterior wall thickness at end-diastole
- SV:
-
Stroke volume
- ESV:
-
End-systole volume
- EDV:
-
End-diastole volume
- CO:
-
Cardiac output
- EF:
-
Ejection fraction
- FS:
-
Fractional shortening
- PCNA:
-
Proliferating cell nuclear antigen
- VEGF:
-
Vascular endothelial growth factor
- HUVEC:
-
Human umbilical vein endothelial cell
- RPMI:
-
Roswell Park Memorial Institute
- FBS:
-
Fetal bovine serum
- OGD:
-
Oxygen and glucose deprivation
References
Zhang, C., Zhang, Y., Zhu, H., et al. (2018). MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat cardiac fibroblasts and extracellular matrix deposition in vivo and in vitro. Cellular Signalling, 46, 145–153. https://doi.org/10.1016/j.cellsig.2018.03.005
Ma, Z. G., Yuan, Y. P., Wu, H. M., et al. (2018). Cardiac fibrosis: New insights into the pathogenesis. International Journal of Biological Sciences, 14, 1645–1657. https://doi.org/10.7150/ijbs.28103
Weintraub, R. G., Semsarian, C., & Macdonald, P. (2017). Dilated cardiomyopathy. Lancet, 390, 400–414. https://doi.org/10.1016/S0140-6736(16)31713-5
Gulati, A., Jabbour, A., Ismail, T. F., et al. (2013). Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA, 309, 896–908. https://doi.org/10.1001/jama.2013.1363
Hu, J., Chen, X., Li, P., et al. (2021). Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovascular Diagnosis and Therapy, 11(2), 348–361. https://doi.org/10.21037/cdt-20-1032
Shaihov-Teper, O., Ram, E., Ballan, N., et al. (2021). Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.052009
Aqil, F., Munagala, R., Jeyabalan, J., et al. (2014). Abstract 5407: Milk derived exosomes: Scalable source of biologically active drug delivery nanoparticles. Cancer Research (Chicago, Ill.), 74, 5407. https://doi.org/10.1158/1538-7445.AM2014-5407
Izumi, H., Kosaka, N., Shimizu, T., et al. (2012). Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. Journal of Dairy Science, 95, 4831–4841. https://doi.org/10.3168/jds.2012-5489
Zhang, C., Zhang, C., Xu, Y., et al. (2020). Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neuroscience Letters, 739, 135399. https://doi.org/10.1016/j.neulet.2020.135399
Sun, J., Shen, H., Shao, L., et al. (2020). HIF-1alpha overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Research & Therapy, 11, 373. https://doi.org/10.1186/s13287-020-01881-7
Matthew R., Warren Chenzhen, Zhang Armin, Vedadghavami Krister, Bokvist Pradeep K., Dhal Ambika G., Bajpayee (2021) Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomaterials Science 9(12) 4260–4277 https://doi.org/10.1039/D0BM01497D
Han, D., Kim, H. Y., Lee, H. J., et al. (2007). Wound healing activity of gamma-aminobutyric acid (GABA) in rats. Journal of Microbiology and Biotechnology, 17, 1661–1669.
Wang, N., Chen, C., Yang, D., et al. (2017). Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1863, 2085–2092. https://doi.org/10.1016/j.bbadis.2017.02.023
Mathew, S. A., Naik, C., Cahill, P. A., et al. (2020). Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cellular and Molecular Life Sciences, 77, 253–265. https://doi.org/10.1007/s00018-019-03268-1
Xiao, Y., Liu, Y., Liu, J., et al. (2018). The association between myocardial fibrosis and depressed capillary density in rat model of left ventricular hypertrophy. Cardiovascular Toxicology, 18, 304–311. https://doi.org/10.1007/s12012-017-9438-7
Krisp, C., Jacobsen, F., McKay, M. J., et al. (2013). Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics, 13, 2670–2681. https://doi.org/10.1002/pmic.201200502
Yoshitomi, Y., Ikeda T., Saito-Takatsuji, H., et al. (2021). Emerging role of AP-1 transcription factor JunB in angiogenesis and vascular development.International Journal of Molecular Sciences, 22.https://doi.org/10.3390/ijms22062804.
Kholia, S., Ranghino, A., Garnieri, P., et al. (2016). Extracellular vesicles as new players in angiogenesis. Vascular Pharmacology, 86, 64–70. https://doi.org/10.1016/j.vph.2016.03.005
Silvestre, J. S., Smadja, D. M., & Levy, B. I. (2013). Postischemic revascularization: From cellular and molecular mechanisms to clinical applications. Physiological Reviews, 93, 1743–1802. https://doi.org/10.1152/physrev.00006.2013
Teng, X., Chen, L., Chen, W., et al. (2015). Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cellular Physiology and Biochemistry, 37, 2415–2424. https://doi.org/10.1159/000438594
Zheng, X., Hermann, D. M., Bahr, M., et al. (2021). The role of small extracellular vesicles in cerebral and myocardial ischemia-molecular signals, treatment targets, and future clinical translation. Stem Cells, 39, 403–413. https://doi.org/10.1002/stem.3329
Li, Q., Song, Y., Wang, Q., et al. (2021). Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics, 11, 3916–3931. https://doi.org/10.7150/thno.52496
Ma, T., Chen, Y., Chen, Y., et al. (2018). MicroRNA-132, Delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int, 2018, 3290372. https://doi.org/10.1155/2018/3290372
Alvarez-Erviti, L., Seow, Y., Yin, H., et al. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29, 341–345. https://doi.org/10.1038/nbt.1807
Hagiwara, K., Ochiya, T., & Kosaka, N. (2014). A paradigm shift for extracellular vesicles as small RNA carriers: From cellular waste elimination to therapeutic applications. Drug Delivery and Translational Research, 4, 31–37. https://doi.org/10.1007/s13346-013-0180-9
Somiya, M., Yoshioka, Y., & Ochiya, T. (2018). Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles, 7, 1440132. https://doi.org/10.1080/20013078.2018.1440132
Liao, Y., Du, X., Li, J., et al. (2017). Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Molecular Nutrition & Food Research, 61.https://doi.org/10.1002/mnfr.201700082.
Zeng, B., Chen, T., Xie, M. Y., et al. (2019). Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. Journal of Dairy Science, 102, 6726–6737. https://doi.org/10.3168/jds.2019-16257
Rani, P., Vashisht, M., Golla, N., et al. (2017). Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. Journal of Functional Foods, 34, 431–439. https://doi.org/10.1016/j.jff.2017.05.009
Tome-Carneiro, J., Fernandez-Alonso, N., Tomas-Zapico, C., et al. (2018). Breast milk microRNAs harsh journey towards potential effects in infant development and maturation. Lipid encapsulation can help. Pharmacological Research, 132, 21–32. https://doi.org/10.1016/j.phrs.2018.04.003
Yamashita, T., Takahashi, Y., & Takakura, Y. (2018). Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biological & Pharmaceutical Bulletin, 41, 835–842. https://doi.org/10.1248/bpb.b18-00133
Lin, D., Chen, T., Xie, M., et al. (2020). Oral administration of bovine and porcine milk exosome alter miRNAs profiles in piglet serum. Science and Reports, 10, 6983. https://doi.org/10.1038/s41598-020-63485-8
Nordgren, T. M., Heires, A. J., Zempleni, J., et al. (2019). Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. Journal of Nutritional Biochemistry, 64, 110–120. https://doi.org/10.1016/j.jnutbio.2018.10.017
Zhou, F., Paz, H. A., Sadri, M., et al. (2019). Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. American Journal of Physiology. Gastrointestinal and Liver Physiology, 317, G618–G624. https://doi.org/10.1152/ajpgi.00160.2019
Martin, C., Patel, M., Williams, S., et al. (2018). Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immunity, 24, 278–284. https://doi.org/10.1177/1753425918785715
Leiferman, A., Shu, J., Grove, R., et al. (2018). A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. Journal of Nutritional Biochemistry, 59, 123–128. https://doi.org/10.1016/j.jnutbio.2018.06.007
Kosaka, N., Izumi, H., Sekine, K., et al. (2010). microRNA as a new immune-regulatory agent in breast milk. Silence, 1, 7. https://doi.org/10.1186/1758-907X-1-7
Benmoussa, A., Ly, S., Shan, S. T., et al. (2017). A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. Journal of Extracell Vesicles, 6, 1401897. https://doi.org/10.1080/20013078.2017.1401897
Svenningsen, P., Sabaratnam, R., & Jensen, B. L. (2020). Urinary extracellular vesicles: Origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Psychologica, 228, e13346. https://doi.org/10.1111/apha.13346
Benmoussa, A., & Provost, P. (2019). Milk microRNAs in Health and disease. Comprehensive Reviews in Food Science and Food Safety, 18, 703–722. https://doi.org/10.1111/1541-4337.12424
Sanwlani, R., Fonseka, P., Chitti, S. V., et al. (2020) Milk-derived extracellular vesicles in inter-organism, cross-species communication and drug delivery. Proteomes, 8.https://doi.org/10.3390/proteomes8020011.
Weiskirchen, R., Weiskirchen, S., & Tacke, F. (2019). Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Molecular Aspects of Medicine, 65, 2–15. https://doi.org/10.1016/j.mam.2018.06.003
Tong, L., Hao, H., Zhang, Z., et al. (2021). Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics, 11, 8570–8586. https://doi.org/10.7150/thno.62046
Acknowledgements
We thank the Department of Medical Ultrastructure, School of Basic Medicine, and the Lab of Biomedical Electronic Microscopy of Higher Research Center, Central South University for assistance with TEM work.
Funding
This research was funded by the Natural Science Foundation of Hunan Province, grant number 2019JJ50936, 2019JJ50950, and the Youth Science Foundation of Xiangya Hospital, grant number 2019Q14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human subjects/informed consent statement
No human studies were carried out by the authors for this article.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Lu, X., Hu, J. et al. Bovine Milk Exosomes Alleviate Cardiac Fibrosis via Enhancing Angiogenesis In Vivo and In Vitro. J. of Cardiovasc. Trans. Res. 15, 560–570 (2022). https://doi.org/10.1007/s12265-021-10174-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10174-0