Abstract
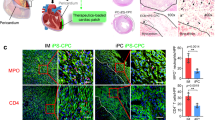
Cardiac patches can help to restore the electrophysiological properties of the heart after myocardial infarction. However, scaffolds for the repair of heart muscle typically require surgical implantation or, if they are injectable, they are not electrically conductive or do not maintain their shape or function. Here, we report the performance, as demonstrated for the repair of infarcted heart muscle in rats and minipigs, of injectable and conductive scaffolds consisting of methacrylated elastin and gelatin, and carbon nanotubes that display shape-memory behaviour, a hierarchical porous structure and a negligible Poisson’s ratio. In rats, the implantation of cell-free patches or patches seeded with rat cardiomyocytes onto the myocardium after ligation of the left anterior descending coronary artery led to functional repair after 4 weeks, as indicated by increases in fractional shortening and the ejection fraction, and by a decrease in the infarcted area. We also observed measures of functional recovery in minipigs with infarcted hearts after the delivery of cell-free patches or patches incorporating cardiomyocytes differentiated from human pluripotent stem cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request.
References
McMurray, J. J. & Pfeffer, M. A. Heart failure. Lancet 365, 1877–1889 (2005).
Matsa, E., Sallam, K. & Wu, J. C. Cardiac stem cell biology: glimpse of the past, present, and future. Circ. Res. 114, 21–27 (2014).
Cates, A. W., Smith, W. M., Ideker, R. E. & Pollard, A. E. Purkinje and ventricular contributions to endocardial activation sequence in perfused rabbit right ventricle. Am. J. Physiol. Heart Circ. Physiol. 281, H490–H505 (2001).
Karikkineth, B. C. & Zimmermann, W. H. Myocardial tissue engineering and heart muscle repair. Curr. Pharm. Biotechnol. 14, 4–11 (2013).
Zimmermann, W. H., Melnychenko, I. & Eschenhagen, T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 25, 1639–1647 (2004).
Breckwoldt, K., Weinberger, F. & Eschenhagen, T. Heart regeneration. Biochim. Biophys. Acta 1863, 1749–1759 (2016).
Rodness, J. et al. VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater. 45, 169–181 (2016).
Ye, L., Zimmermann, W. H., Garry, D. J. & Zhang, J. Y. Patching the heart cardiac repair from within and outside. Circ. Res. 113, 922–932 (2013).
Xiong, Q. et al. Bioenergetic and functional consequences of cellular therapy activation of endogenous cardiovascular progenitor cells. Circ. Res. 111, 455–468 (2012).
Xiong, Q. et al. Functional consequences of human induced pluripotent stem cell therapy myocardial ATP turnover rate in the in vivo swine heart with postinfarction remodeling. Circulation 127, 997–1008 (2013).
Yang, J.-A., Yeom, J., Hwang, B. W., Hoffman, A. S. & Hahn, S. K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 39, 1973–1986 (2014).
Zhao, S. et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction. Nat. Commun. 7, 13306 (2016).
Shin, M., Song, K.H., Burrell, J. C., Cullen, D. K. & Burdick, J. A. Injectable and conductive granular hydrogels for 3D printing and electroactive tissue support. Adv. Sci. 6, 1901229.
Wang, L. L. et al. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat. Biomed. Eng. 1, 983–992 (2017).
Zhou, J. et al. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics 8, 3317–3330 (2018).
Cui, Z. et al. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 8, 2752–2764 (2018).
Bencherif, S. A. et al. Injectable preformed scaffolds with shape-memory properties. Proc. Natl Acad. Sci. USA 109, 19590–19595 (2012).
Montgomery, M. et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 16, 1038–1046 (2017).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Lee, J. M. et al. Nanoenabled direct contact interfacing of syringe-injectable mesh electronics. Nano Lett. 19, 5818–5826 (2019).
Shah, N. J. et al. An injectable bone marrow–like scaffold enhances T cell immunity after hematopoietic stem cell transplantation. Nat. Biotechnol. 37, 293–302 (2019).
Kim, I., Lee, S. S., Bae, S., Lee, H. & Hwang, N. S. Heparin functionalized injectable cryogel with rapid shape-recovery property for neovascularization. Biomacromolecules 19, 2257–2269 (2018).
Kim, M., Choe, Y. & Kim, G. Injectable hierarchical micro/nanofibrous collagen-based scaffolds. Chem. Eng. J. 365, 220–230 (2019).
Zhao, X., Guo, B., Wu, H., Liang, Y. & Ma, P. X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 9, 2784 (2018).
Qiu, Y. et al. A role for matrix stiffness in the regulation of cardiac side population cell function. Am. J. Physiol. Heart Circ. Physiol. 308, H990–H997 (2015).
Annabi, N. et al. Highly elastic and conductive human-based protein hybrid hydrogels. Adv. Mater. 28, 40–49 (2016).
Kharaziha, M. et al. Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 35, 7346–7354 (2014).
Hwang, J. Y., Kim, H. S., Kim, J. H., Shin, U. S. & Lee, S. H. Carbon nanotube nanocomposites with highly enhanced strength and conductivity for flexible electric circuits. Langmuir 31, 7844–7851 (2015).
Martinelli, V. et al. Carbon nanotubes instruct physiological growth and functionally mature syncytia: nongenetic engineering of cardiac myocytes. ACS Nano 7, 5746–5756 (2013).
Martinelli, V. et al. Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 12, 1831–1838 (2012).
Shin, S. R. et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 7, 2369–2380 (2013).
Shin, S. R. et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 6, 362–372 (2012).
Wu, Y., Wang, L., Guo, B. & Ma, P. X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano 11, 5646–5659 (2017).
Kucheyev, S. O. et al. Super-compressibility of ultralow-density nanoporous silica. Adv. Mater. 24, 776–780 (2012).
Sun, J. Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Lv, S. et al. Designed biomaterials to mimic the mechanical properties of muscles. Nature 465, 69–73 (2010).
Yeo, G. C. et al. Fabricated Elastin. Adv. Healthc. Mater. 4, 2530–2556 (2015).
Liu, Y. et al. Highly flexible and resilient elastin hybrid cryogels with shape memory, injectability, conductivity, and magnetic responsive properties. Adv. Mater. 28, 7758–7767 (2016).
Koshy, S. T., Ferrante, T. C., Lewin, S. A. & Mooney, D. J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 35, 2477–2487 (2014).
He, S. et al. Preservation of conductive propagation after surgical repair of cardiac defects with a bio-engineered conductive patch. J. Heart Lung Transpl. 37, 912–924 (2018).
Kong, W. et al. Optical measurements of intramural action potentials in isolated porcine hearts using optrodes. Heart Rhythm 4, 1430–1436 (2007).
Pleger, S. T. et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci. Transl. Med. 3, 92ra64 (2011).
Wang, L. Y. et al. Mussel-Inspired conductive cryogel as cardiac tissue patch to repair myocardial infarction by migration of conductive nanoparticles. Adv. Funct. Mater. 26, 4293–4305 (2016).
Li, X. et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 35, 5679–5688 (2014).
Kapnisi, M. et al. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv. Funct. Mater. 28, 1800618 (2018).
Liang, S. et al. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv. Mater. 30, e1704235 (2018).
He, Y. T. et al. Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics 8, 5159–5177 (2018).
Hulsmans, M. et al. Macrophages facilitate electrical conduction in the heart. Cell 169, 510–522 (2017).
Dick, S. A. et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 664–664 (2019).
Ong, S. B. et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 186, 73–87 (2018).
Zhang, F. X. et al. Transplantation of iPSc ameliorates neural remodeling and reduces ventricular arrhythmias in a post-infarcted swine model. J. Cell. Biochem. 115, 531–539 (2014).
Arana, M. et al. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials 35, 143–151 (2014).
Gao, L. et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 137, 1712 (2018).
Perea-Gil, I. et al. A cell-enriched engineered myocardial graft limits infarct size and improves cardiac function: pre-clinical study in the porcine myocardial infarction model. JACC Basic Transl. Sci. 1, 360–372 (2016).
Kawamura, M. et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–S37 (2012).
Nichol, J. W. et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010).
Harsdorf, R., von., Li, P. F. & Dietz, R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99, 2934–2941 (1999).
Lin, B. et al. Culture in glucose-depleted medium supplemented with fatty acid and 3,3′,5-triiodo-l-thyronine facilitates purification and maturation of human pluripotent stem cell-derived cardiomyocytes. Front. Endocrinol. 8, 253 (2017).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (nos U1601221, 31922043 and 31572343), Guangdong Province Science and Technology Projects (2016B090913004). M.M.Q.X. and K.M thank NSERC Discovery grants and NSERC Discovery Accelerator Supplements (DAS) Awards for supporting this work.
Author information
Authors and Affiliations
Contributions
M.M.Q.X. and X.Q. conceived the research. X.Q. and M.M.Q.X. supervised the project and provided research direction, including all experimental designs. Y.L., M.M.Q.X. and L.W. synthesized and characterized the materials. L.W., Y.L., X.Q., M.M.Q.X., B.L., G.Y. and Y.H. performed the in vitro experiments of the engineered cardiac patches. L.W., G.Y., Y.H., Y.G., B.G. and X.Q. performed the in vivo experiments. K.M. provided critical input that shaped the research, data analysis and manuscript revision. L.W., Y.L., X.Q. and M.M.Q.X. verified data integrity and performed the statistical analyses. M.M.Q.X., L.W., Y.L., K.M., B.L. and X.Q. interpreted the data and co-wrote the manuscript. All of the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Milica Radisic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, Tables 1–17 and References, and the captions for Supplementary Videos 1–10.
Supplementary Video 1
Rigid CNTs bridged by extremely flexible biomacromolecular coils.
Supplementary Video 2
The compressive elasticity and water-driven shape memory behaviour of the EGC20 scaffold.
Supplementary Video 3
The loading, injection and instant-recovery process of an EGC20 patch injected out of the pipette tip.
Supplementary Video 4
Minimally invasive delivery of an EGC20 patch onto porcine heart through a catheter.
Supplementary Video 5
Fibrin-glue delivery under a simulative thoracoscope trainer.
Supplementary Video 6
Calcium transients in rat CMs cultured in different scaffolds on day 7.
Supplementary Video 7
Synchronous contraction of an EGC20 cardiac patch seeded with rat cardiomyocytes in vitro on day 7.
Supplementary Video 8
HECP injected and fixed on the infarcted heart of a porcine MI model.
Supplementary Video 9
LV at the papillary-muscle level in the study groups, captured by echocardiography.
Supplementary Video 10
Beating activity of hPSC-differentiated cardiomyocytes.
Rights and permissions
About this article
Cite this article
Wang, L., Liu, Y., Ye, G. et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat Biomed Eng 5, 1157–1173 (2021). https://doi.org/10.1038/s41551-021-00796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00796-9
This article is cited by
-
Two way workable microchanneled hydrogel suture to diagnose, treat and monitor the infarcted heart
Nature Communications (2024)
-
Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications
Military Medical Research (2023)
-
Application of biomedical materials in the diagnosis and treatment of myocardial infarction
Journal of Nanobiotechnology (2023)
-
Water-responsive supercontractile polymer films for bioelectronic interfaces
Nature (2023)
-
Chronological adhesive cardiac patch for synchronous mechanophysiological monitoring and electrocoupling therapy
Nature Communications (2023)