Abstract
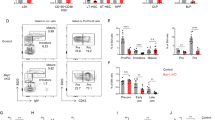
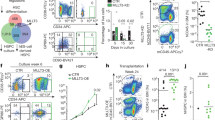
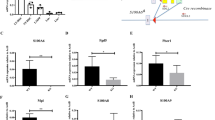
Little is known of hematopoietic stem (HSC) and progenitor (HPC) cell self-renewal. The role of Brahma (BRM), a chromatin remodeler, in HSC function is unknown. Bone marrow (BM) from Brm−/− mice manifested increased numbers of long- and short-term HSCs, GMPs, and increased numbers and cycling of functional HPCs. However, increased Brm−/− BM HSC numbers had decreased secondary and tertiary engraftment, suggesting BRM enhances HSC self-renewal. Valine was elevated in lineage negative Brm−/− BM cells, linking intracellular valine with Brm expression. Valine enhanced HPC colony formation, replating of human cord blood (CB) HPC-derived colonies, mouse BM and human CB HPC survival in vitro, and ex vivo expansion of normal mouse BM HSCs and HPCs. Valine increased oxygen consumption rates of WT cells. BRM through CD98 was linked to regulated import of branched chain amino acids, such as valine, in HPCs. Brm−/− LSK cells exhibited upregulated interferon response/cell cycle gene programs. Effects of BRM depletion are less apparent on isolated HSCs compared to HSCs in the presence of HPCs, suggesting cell extrinsic effects on HSCs. Thus, intracellular valine is regulated by BRM expression in HPCs, and the BRM/valine axis regulates HSC and HPC self-renewal, proliferation, and possibly differentiation fate decisions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12.
Shaheen M, Broxmeyer HE. Cytokine/receptor families and signal transduction. In: Hoffman R, Benz E, Silberstein LE, Heslop H, Weitz J, Anastasi J (eds). Hematology: basic principles and practice. 7th ed. Elsevier, Philadelphia, 2017. pp. 163–75.
S Shaheen M, Broxmeyer HE. Principles of cytokine signaling. In: Hoffman R, Benz E, Silberstein LE, Heslop H, Weitz J, Anastasi J (eds). Hematology: basic principles and practice. 6th ed. Elsevier Saunders, Philadelphia, 2013. pp. 136–46.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34.
Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–13.
Broxmeyer HE, Lee M-R, Hangoc G, Cooper S, Prasain N, Kim Y-J, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–7.
Carow CE, Hangoc G, Broxmeyer HE. Human multipotential progenitor cells (CFU-GEMM) have extensive replating capacity for secondary CFU-GEMM: an effect enhanced by cord blood plasma. Blood. 1993;81:942–9.
Carow CE, Hangoc G, Cooper SH, Williams DE, Broxmeyer HE. Mast cell growth factor (c-kit ligand) supports the growth of human multipotential progenitor cells with a high replating potential. Blood. 1991;78:2216–21.
Kwon CS, Wagner D. Unwinding chromatin for development and growth: a few genes at a time. Trends Genet. 2007;23:403–12.
Muchardt C, Yaniv M. When the SWI/SNF complex remodels…the cell cycle. Oncogene. 2001;20:3067–75.
Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420.
Yoo AS, Crabtree GR. ATP-dependent chromatin remodeling in neural development. Curr Opin Neurobiol. 2009;19:120–6.
Raab JR, Runge JS, Spear CC, Magnuson T. Co-regulation of transcription by BRG1 and BRM, two mutually exclusive SWI/SNF ATPase subunits. Epigenetics Chromatin. 2017;10:62.
Sif S, Saurin AJ, Imbalzano AN, Kingston RE. Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev. 2001;15:603–18.
Stanton BZ, Hodges C, Calarco JP, Braun SMG, Ku WL, Kadoch C, et al. Smarca4 ATPase mutations disrupt direct eviction of PRC1 from chromatin. Nat Genet. 2017;49:282–8.
Wong AK, Shanahan F, Chen Y, Lian L, Ha P, Hendricks K, et al. BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res. 2000;60:6171–7.
Bottardi S, Ross J, Pierre-Charles N, Blank V, Milot E. Lineage-specific activators affect beta-globin locus chromatin in multipotent hematopoietic progenitors. EMBO J. 2006;25:3586–95.
Bultman S, Gebuhr T, Yee D, Mantia CL, Nicholson J, Gilliam A, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–95.
Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008;135:493–500.
Kidder BL, Palmer S, Knott JG. SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells. 2009;27:317–28.
Singhal N, Esch D, Stehling M, Scholer HR. BRG1 is required to maintain pluripotency of murine embryonic stem cells. Biores Open Access. 2014;3:1–8.
Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha). EMBO J. 1998;17:6979–91.
Naidu SR, Love IM, Imbalzano AN, Grossman SR, Androphy EJ. The SWI/SNF chromatin remodeling subunit BRG1 is a critical regulator of p53 necessary for proliferation of malignant cells. Oncogene. 2009;28:2492–501.
Kadam S, Emerson BM. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol Cell. 2003;11:377–89.
Filippi MD, Ghaffari S. Mitochondria in the maintenance of hematopoietic stem cells: new perspectives and opportunities. Blood. 2019;133:1943–52.
Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161:1553–65.
Snoeck HW. Mitochondrial regulation of hematopoietic stem cells. Curr Opin Cell Biol. 2017;49:91–8.
Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–4.
Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–63.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54.
Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–8.
Taya Y, Ota Y, Wilkinson AC, Kanazawa A, Watarai H, Kasai M, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354:1152–5.
Honda M, Takehana K, Sakai A, Tagata Y, Shirasaki T, Nishitani S, et al. Malnutrition impairs interferon signaling through mTOR and FoxO pathways in patients with chronic Hepatitis C. Gastroenterology. 2011;141:128–e2.
Broxmeyer HE, Christopherson K, Hangoc G, Cooper S, Mantel C, Renukaradhya GJ, et al. CD1d expression on and regulation of murine hematopoietic stem and progenitor cells. Blood. 2012;119:5731–41.
Broxmeyer HE, Hangoc G, Cooper S, Ribeiro RC, Graves V, Yoder M, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. PNAS. 1992;89:4109–13.
Yu RY, Wang X, Pixley FJ, Yu JJ, Dent AL, Broxmeyer HE, et al. BCL-6 negatively regulates macrophage proliferation by suppressing autocrine IL-6 production. Blood. 2005;105:1777–84.
Broxmeyer HE, Hoggatt J, O’Leary HA, Mantel C, Chitteti BR, Cooper S, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–96.
Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629–32.
Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21:580–9.
Soga T, Heiger DN. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem. 2000;72:1236–41.
Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res. 2003;2:488–94.
Soga T, Ueno Y, Naraoka H, Ohashi Y, Tomita M, Nishioka T. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem. 2002;74:2233–9.
Overmyer KA, Evans CR, Qi NR, Minogue CE, Carson JJ, Chermside-Scabbo CJ, et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 2015;21:468–78.
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95.
Anso E, Weinberg SE, Diebold LP, Thompson BJ, Malinge S, Shumacker PT, et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat Cell Biol. 2017;19:614–25.
Ito K, Turcotte R, Cui J, Zimmerman SE, Pinho S, Mizoguchi T, et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science. 2016;354:1156–60.
Luchsinger LL, de Almeida MJ, Corrigan DJ, Mumau M, Snoeck HW. Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature. 2016;529:528–31.
Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–7.
Acknowledgements
These studies were supported by the Public Health Service Grants from the NIH to HEB: R35 HL139599 (Outstanding Investigator Award), R01 DK109188, and U54 DK106846. JR was supported as a postdoctoral fellow by NIH T32 DK007519 (PI HEB). We thank Dr. Ching-Pin Chang for Brm−/− mice.
Author information
Authors and Affiliations
Contributions
SRN conceived the project idea and hypothesized the stem cell phenotype in Brm−/− mice, performed metabolism experiments and connected BRM to intracellular valine. MC and SC performed and analyzed in vitro and ex vivo assays characterizing BRM and valine regulation of HSC/HPC including colony assays, phenotyping and expansion assays. MC, SC, and JR performed in vivo analyses of BRM and valine regulation of HSC/HPC. JR performed and analyzed RNA-sequencing and CD98 expression analyses. JR and MC interpreted data and hypothesized the working model connecting valine to BRM in HSC/HPC mechanistically and MC, JR, and SC designed and performed the ex vivo and in vivo experiments to validate this model. XH assisted and performed OCR experiments. SRN, MC, JR, and HEB wrote the paper and drafts of the paper were evaluated by all co-authors. HEB designed experiments, scored colony assays, supervised the study, and acquired funding.
Corresponding authors
Ethics declarations
Competing interests
HEB is on the Scientific Advisory Board of Elixell Corp, a stem cell company. None of the other authors have conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Naidu, S.R., Capitano, M., Ropa, J. et al. Chromatin remodeling subunit BRM and valine regulate hematopoietic stem/progenitor cell function and self-renewal via intrinsic and extrinsic effects. Leukemia 36, 821–833 (2022). https://doi.org/10.1038/s41375-021-01426-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01426-8
This article is cited by
-
SWI/SNF complexes in hematological malignancies: biological implications and therapeutic opportunities
Molecular Cancer (2023)
-
Cell-intrinsic factors governing quiescence vis-à-vis activation of adult hematopoietic stem cells
Molecular and Cellular Biochemistry (2023)