Attempts to Access a Series of Pyrazoles Lead to New Hydrazones with Antifungal Potential against Candida species including Azole-Resistant Strains
Abstract
:1. Introduction
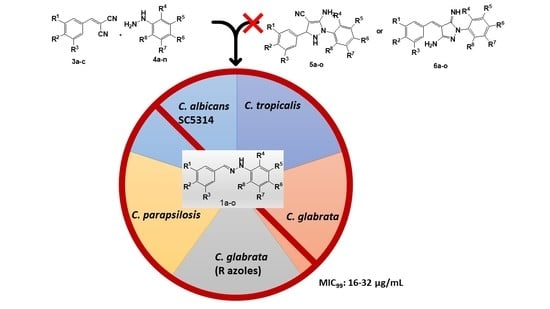
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Synthesis of Benzylidenemalononitriles (3a–c)
3.1.1. 2-(3,4,5-trimethoxybenzylidene)malononitrile (3a)
3.1.2. 2-(4-nitrobenzylidene)malononitrile (3b)
3.1.3. 2-(4-bromobenzylidene)malononitrile (3c)
3.2. General Procedure for the Preparation of Hydrazone Derivatives (1a–o)
3.2.1. 1-(2-(trifluoromethyl)phenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1a)
3.2.2. 1-(2-methoxyphenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1b)
3.2.3. 1-(2-bromophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1c)
3.2.4. 1-(2-chlorophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1d)
3.2.5. 1-(2,4-dichlorophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1e)
3.2.6. 1-(2,4-difluorophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1f)
3.2.7. 1-(o-tolyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1g)
3.2.8. 1-(4-chlorophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1h)
3.2.9. 1-(2-fluorophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1i)
3.2.10. 1-(pentafluorophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1j)
3.2.11. 1-(4-bromophenyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1k)
3.2.12. 1-(p-tolyl)-2-(3,4,5-trimethoxybenzylidene)hydrazine (1l)
3.2.13. 1-(2-methoxyphenyl)-2-(4-nitrobenzylidene)hydrazine (1m)
3.2.14. 1-(4-bromobenzylidene)-2-phenylhydrazine (1n)
3.2.15. 1-(4-bromobenzylidene)-2-(3,4-dimethylphenyl)hydrazine (1o)
3.3. X-ray Crystallography
3.4. MIC99 Determination Assays
3.5. Cell Viability Assay
3.6. Cancer Cell Proliferation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. First Meeting of the WHO Antifungal Expert Group on Identifying Priority Fungal Pathogens. Available online: https://www.who.int/publications/i/item/9789240006355 (accessed on 19 July 2021).
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. 2012, 18, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012, 18, 38–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datry, A.; Bart-Delabesse, E. Caspofungin: Mode of action and therapeutic applications. Rev. Med. Interne 2006, 27, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.C.; Despaigne, A.A.R.; Da Silva, J.G.; Silva, N.F.; Vilela, C.F.; Mendes, I.C.; Takahashi, J.A.; Beraldo, H. Structural Studies and Investigation on the Activity of Imidazole-Derived Thiosemicarbazones and Hydrazones against Crop-Related Fungi. Molecules 2013, 18, 12645–12662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, D.M.; Cammarata, A.; Backes, G.; Palmer, G.E.; Jursic, B.S. Synthesis and antifungal activity of substituted 2,4,6-pyrimidinetrione carbaldehyde hydrazones. Bioorg. Med. Chem. 2014, 22, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bizzarri, B.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; D’Ascenzio, M.; Lilli, D.; Rivanera, D. Synthesis and biological evaluation of novel 2,4-disubstituted-1,3-thiazoles as anti-Candida spp. Agents. Eur. J. Med. Chem. 2011, 46, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Nelson, R.; Kesternich, V.; Perez-Fehrmann, M.; Christen, P.; Marcourt, L. Synthesis and Antifungal Activity of Diaryl Hydrazones from 2,4-Dinitrophenylhydrazine. J. Chil. Chem. Soc. 2016, 61, 3081–3084. [Google Scholar] [CrossRef] [Green Version]
- Gouhar, R.S.; Fathalla, O.A.; Abd El-Karim, S.S. Synthesis and anticancer screening of some novel substituted pyrazole derivatives. Der Pharma Chem. 2013, 5, 225–233. [Google Scholar]
- Kulkarni, A.; Quang, P.; Curry, V.; Keyes, R.; Zhou, W.; Cho, H.; Baffoe, J.; Török, B.; Stieglitz, K. 1,3-disubstituted-4-aminopyrazolo [3, 4-d] pyrimidines, a new class of potent inhibitors for phospholipase D. Chem. Biol. Drug Des. 2014, 84, 270–281. [Google Scholar] [CrossRef]
- Attar, S.R.; Shinde, B.; Kamble, S.B. Enhanced catalytic activity of bio-fabricated ZnO NPs prepared by ultrasound-assisted route for the synthesis of tetraketone and benzylidenemalonitrile in hydrotropic aqueous medium. Res. Chem. Intermed. 2020, 46, 4723–4748. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, X.; Cheng, Q.; Zhang, T.; Luo, J. An efficient and recyclable acid–base bifunctional core–shell nano-catalyst for the one-pot deacetalization–Knoevenagel tandem reaction. New J. Chem. 2018, 42, 11610–11615. [Google Scholar]
- Yamashita, K.; Tanaka, T.; Hayashi, M. Use of isopropyl alcohol as a solvent in Ti(O-i-Pr)4-catalyzed Knöevenagel reactions. Tetrahedron 2005, 61, 7981–7985. [Google Scholar] [CrossRef]
- Khan, M.; Ahad, G.; Manaf, A.; Naz, R.; Hussain, S.R.; Deeba, F.; Shah, S.; Khan, A.; Ali, M.; Zaman, K.; et al. Synthesis, in vitro urease inhibitory activity, and molecular docking studies of (perfluorophenyl)hydrazone derivatives. Med. Chem. Res. 2019, 28, 873–883. [Google Scholar] [CrossRef]
- Buzykin, B.I. Synthesis and spectral characteristics of 1,4-diaryl-2-arylidenebenzhydrazidines. Izv. Akad. Nauk. SSSR Seriya Khimicheskaya 1983, 7, 1588–1593. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System; Version 1.171.38.46; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, R.B. The NCI In Vitro Anticancer Drug Discovery Screen; Humana Press Inc.: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]







| MIC Values (µg/mL) on Candida spp. [a,b,c,d] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Compound | C. albicans SC5314 | C. dubliniensis | C. glabrata | C. parapsilosis | C. tropicalis | C. albicans (Mucoviscidosis) | C. albicans (R echinocandins) | C. glabrata (R azoles) |
| 1 | 1c | 32 | >32 [e] | 32 | 32 | >32 | >32 | >32 | 32 |
| 2 | 1d | 32 | >32 | 16 | >32 | >32 | >32 | >32 | 32 |
| 3 | 1i | 32 | >32 | 32 | 32 | >32 | >32 | >32 | 32 |
| 4 | 1k | 32 | >32 | 16 | 32 | 32 | >32 | >32 | >32 |
| 5 | 1l | 32 | >32 | 16 | 32 | 32 | >32 | >32 | 32 |
| 6 | Fluconazole | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | >32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negru, G.; Kamus, L.; Bîcu, E.; Shova, S.; Sendid, B.; Dubar, F.; Ghinet, A. Attempts to Access a Series of Pyrazoles Lead to New Hydrazones with Antifungal Potential against Candida species including Azole-Resistant Strains. Molecules 2021, 26, 5861. https://doi.org/10.3390/molecules26195861
Negru G, Kamus L, Bîcu E, Shova S, Sendid B, Dubar F, Ghinet A. Attempts to Access a Series of Pyrazoles Lead to New Hydrazones with Antifungal Potential against Candida species including Azole-Resistant Strains. Molecules. 2021; 26(19):5861. https://doi.org/10.3390/molecules26195861
Chicago/Turabian StyleNegru, Georgiana, Laure Kamus, Elena Bîcu, Sergiu Shova, Boualem Sendid, Faustine Dubar, and Alina Ghinet. 2021. "Attempts to Access a Series of Pyrazoles Lead to New Hydrazones with Antifungal Potential against Candida species including Azole-Resistant Strains" Molecules 26, no. 19: 5861. https://doi.org/10.3390/molecules26195861