Common Shortcomings in Study on Radiopharmaceutical Design Research: A Case Study of 99mTc-Labelled Methotrexate
Abstract
:1. Introduction
2. Methotrexate Labelling with Technetium-99m Case Study
2.1. Direct Method of the Syntheses of [99mTc]Tc-MTX Complex in Scientific Literature
2.2. Direct Methods of Syntheses of the [99mTc]Tc-Intermediate Complexes in Scientific Literature
2.3. Own Results of Syntheses of the 99mTc-Intermediate Complexes and the Final [99mTc]Tc-MTX Complex
2.3.1. [99mTc]Tc-MTX Radiocomplex Synthesis
2.3.2. Properties Determination of 99mTc-Intermediate and [99mTc]Tc-MTX Complexes
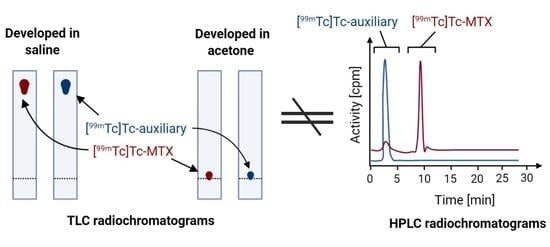
2.3.3. Discussion of Results
3. Recommendations and Indications
3.1. Planar Chromatography Methods Validation in Radiopharmaceutical Chemistry
3.2. Recommendations for Radiopreparation Stability Studies in Radiopharmaceutical Chemistry
3.3. Recommendations for Radiopreparation Lipophilicity Studies in Radiopharmaceutical Chemistry
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| EDTA | ethylenediaminetetraacetic acid |
| FA | folic acid |
| Glu | glutamic acid |
| ITLC | instant thin layer chromatography |
| Lys | lysine |
| MTX | methotrexate |
| PBS | phosphate-buffered saline |
| Rf | retention factor |
| RT | retention time |
| TLC | thin layer chromatography |
References
- Pile, K.D.; Graham, G.G. Methotrexate. In Compendium of Inflammatory Diseases; Parnham, M.J., Ed.; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.-L.; Xu, H.E.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Halik, P.K.; Koźmiński, P.; Gniazdowska, E. Perspectives of Methotrexate-Based Radioagents for Application in Nuclear Medicine. Mol. Pharm. 2021, 18, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mathur, R.; Das, M.; Swarnakar, N.K.; Mishra, A.K. Synthesis, pharmacoscintigraphic evaluation and antitumor efficacy of methotrexate-loaded, folate-conjugated, stealth albumin nanoparticles. Nanomedicine 2011, 6, 1733–1754. [Google Scholar] [CrossRef]
- Dar, U.K.; Khan, I.U.; Javed, M.; Ahmad, F.; Ali, M.; Hyder, S.W. Preparation and biodistribution in mice of a new radiopharmaceutical-technetium-99m labeled methotrexate, as a tumor diagnostic agent. Hell. J. Nucl. Med. 2012, 15, 120–124. [Google Scholar] [PubMed]
- Das, M.; Datir, S.R.; Singh, R.P.; Jain, S. Augmented Anticancer Activity of a Targeted, Intracellularly Activatable, Theranostic Nanomedicine Based on Fluorescent and Radiolabeled, Methotrexate-Folic Acid-Multiwalled Carbon Nanotube Conjugate. Mol. Pharm. 2013, 10, 2543–2557. [Google Scholar] [CrossRef]
- Rasheed, R.; Javed, M.; Ahmad, F.; Sohail, A.; Murad, S.; Masood, M.; Rasheed, S.; Rasheed, S. Preparation of (99m)Tc-labelled methotraxate by a direct labeling technique as a potential diagnostic agent for breast cancer and preliminary clinical results. Hell. J. Nucl. Med. 2013, 16, 33–37. [Google Scholar] [CrossRef]
- Singh, V.K.; Subudhi, B.B. Development of reversible glutamine conjugate of methotrexate for enhanced brain delivery. Med. Chem. Res. 2015, 24, 624–635. [Google Scholar] [CrossRef]
- Singh, V.K.; Subudhi, B.B. Development and characterization of lysine-methotrexate conjugate for enhanced brain delivery. Drug Deliv. 2016, 23, 2327–2337. [Google Scholar] [CrossRef]
- Ozgenc, E.; Ekinci, M.; Ilem-Ozdemir, D.; Gundogdu, E.; Asikoglu, M. Radiolabeling and in vitro evaluation of 99mTc-methotrexate on breast cancer cell line. J. Radioanal. Nucl. Chem. 2016, 307, 627–633. [Google Scholar] [CrossRef]
- Papachristou, M.; Kastis, G.A.; Stavrou, P.Z.; Xanthopoulos, S.; Furenlid, L.R.; Datseris, I.E.; Bouziotis, P. Radiolabeled methotrexate as a diagnostic agent of inflammatory target sites: A proof-of-concept study. Mol. Med. Rep. 2018, 17, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Gillani, J.; Jielani, A.; Irum, F.; Lodhi, N.; Rasheed, S.; Rasheed, S. Tc99m Methotrexate (MTX) A Novel Complex for Imaging of Rheumatoid Arthritis (RA): First Clinical Trials. Gen Med (Los Angel) Pers. Med. 2016, S213, 2016. [Google Scholar] [CrossRef]
- Fleay, R.F.; Grad, A.P. 99mTc-Labelled E.D.T.A. for Renal Scanning. Aust. Radiol. 1968, 12, 265–267. [Google Scholar] [CrossRef]
- Baker, R.J.; Diamanti, C.I.; Goodwin, D.A.; Meares, C.F. Technetium-99 m complexes of EDTA analogs: Studies of the radiochemistry and biodistribution. Int. J. Nucl. Med. Biol. 1981, 8, 159–169. [Google Scholar] [CrossRef]
- Castagnoli, A.; Pupi, A.; Guizzardi, G.; Ferri, P.; Taverni, N.; Meldolesi, U.; Formiconi, A. Brain scintigraphy with 99mTc-citrate and 99mTc-DTPA: A clinical comparison of the results. Eur. J. Nucl. Med. 1982, 7, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Ercan, M.T.; Aras, T.; Ünsal, I.S.; Arikan, Ü.; Ünlenen, E.; Hasçelik, Z. Technetium-99m citrate for imaging inflammation: An experimental study. Med. J. Islamic World Acad. Sci. 1992, 5, 180–188. [Google Scholar]
- Ercan, M.T.; Aras, T.; Ünlenen, E.; Unlü, M.; Ünsal, I.S.; Hasçelik, Z. 99mTc-citrate versus 67Ga-citrate for the scintigraphic visualization of inflammatory lesions. Nucl. Med. Biol. 1993, 20, 881–887. [Google Scholar] [CrossRef]
- Ballinger, J.R.; Cowan, D.S.; Boxen, I.; Zhang, Z.M.; Rauth, A.M. Effect of hypoxia on the accumulation of technetium-99m-glucarate and technetium-99m-gluconate by Chinese hamster ovary cells in vitro. J. Nucl. Med. 1993, 34, 242–245. [Google Scholar]
- El-Ghany, E.A.; Attia, F.F.; Marzouk, F.; El-Kolaly, M.T. Organic synthesis and technetium-99m labeling of some amino-phenol ligands. J. Radioanal. Nucl. Chem. 2000, 245, 237–242. [Google Scholar] [CrossRef]
- Yigit, U.S.; Lambrecht, F.Y.; Unak, P.; Biber, F.Z.; Medine, E.I.; Cetinkaya, B. Preparation of (99m)Tc labeled vitamin C (ascorbic acid) and biodistribution in rats. Chem. Pharm. Bull. 2006, 54, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mamede, A.C.; Abrantes, A.M.; Pires, A.S.; Tavares, S.D.; Serra, M.E.; Maia, J.M.; Botelho, M.F. Radiolabelling of ascorbic acid: A new clue to clarify its action as an anticancer agent? Curr. Radiopharm. 2012, 5, 106–112. [Google Scholar] [CrossRef]
- Park, P.; Kim, Y.J.; Lee, J.Y.; Lee, Y.S.; Jeong, J.M. Imaging of the third gasotransmitter hydrogen sulfide using 99mTc-labeled alpha-hydroxy acids. Nucl. Med. Biol. 2019, 76–77, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kweon, Y.; Kim, Y.J.; Lee, Y.S.; Jeong, J.M. Imaging H2S in hypoxia using [99mTc]Tc-gluconate. J. Nucl. Med. 2020, 61 (Suppl. 1), 1057. [Google Scholar]
- Sabahnoo, H.; Hosseinimehr, S.J. Optimizing Labeling Conditions for Cysteine-Based Peptides with 99mTc. J. Braz. Chem. Soc. 2016, 27, 1966–1973. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Martini, P.A. Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef] [Green Version]
- Safi, B.; Mertens, J.; De Proft, F.; Alberto, R.; Geerlings, P. Relative Stability of Mixed [3 + 1] Tc and Re Complexes: A Computational and Conceptual DFT Study. J. Phys. Chem. A 2005, 109, 1944–1951. [Google Scholar] [CrossRef]
- Chopra, A.; Shan, L.; Eckelman, W.C.; Leung, K.; Menkens, A.E. Important parameters to consider for the characterization of PET and SPECT imaging probes. Nucl. Med. Biol. 2011, 38, 1079–1084. [Google Scholar] [CrossRef]
- Motaleb, M.A.; El-Safoury, D.M.; Abd-Alla, W.H.; Awad, G.A.S.; Sakr, T.M. Radiosynthesis, molecular modeling studies and biological evaluation of 99mTc-Ifosfamide complex as a novel probe for solid tumor imaging, Int. J. Radiat. Biol. 2018, 94, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Moustapha, M.E.; Motaleb, M.A.; Shweeta, H.; Farouk, M. Synthesis and biological evaluation of technetium-sarafloxacin complex for infection imaging. J. Radioanal. Nucl. Chem. 2016, 307, 699–705. [Google Scholar] [CrossRef]
- Motaleb, M.A.; Selim, A.A.; El-Tawoosy, M.; Sanad, M.H.; El-Hashash, M.A. Synthesis, characterization, radiolabeling and biodistribution of a novel cyclohexane dioxime derivative as a potential candidate for tumor imaging. Int. J. Radiat. Biol. 2018, 4, 590–596. [Google Scholar] [CrossRef]











| Syntheses of [99mTc]Tc-MTX (Additional Reagents) | PC Rf Values [Mobile Phase] | HPLC Analysis | logD | Reference | |
|---|---|---|---|---|---|
| [99mTc]Tc-MTX | At origin [acetone] | No data | No data | No data | [5] |
| [99mTc]Tc-MTX (stannous tartrate, AA) | 0.00–0.01 [acetone] | 0.9–1.0 [saline] | No data | −2.22 * | [6] |
| [99mTc]Tc-MTX | No data | No data | No data | No data | [7] |
| [99mTc]Tc-MTX (stannous tartrate, AA, sodium citrate and pyrophosphate) | 0.00–0.01 [acetone] | 0.9–1.0 [saline] | No data | No data ** | [8] |
| [99mTc]Tc-MTX and [99mTc]Tc-MTX-Glu2 | At origin [acetone] | ~1 [Pyr:AcOH:H2O 3:5:1.5] | No data | No data | [9] |
| [99mTc]Tc-MTX and [99mTc]Tc-MTX-Lys2 | At origin [acetone] | ~1 [Pyr:AcOH:H2O 3:5:1.5] | No data | No data | [10] |
| [99mTc]Tc-MTX (AA and sodium citrate) | At origin [acetone] | ~1 [saline] | No data | No data | [13] |
| [99mTc]Tc-MTX (stannous tartrate and AA) | At origin [acetone] | ~1 [ACN/ H2O /TFA; 50/25/1.5] | No data | No data | [11] |
| [99mTc]Tc-MTX (sodium gluconate) | 0.0–0.1 [acetone] | 0.9–1.0 [saline] | Executed | −2.28 ± 0.03 | [12] |
| Syntheses of the 99mTc-Complexes (Reducing Reagents) | PC Rf Values [Mobile Phase] | HPLC RT [min] | logD | Reference | |
|---|---|---|---|---|---|
| [99mTc]Tc-EDTA (SnCl2) | At origin [acetone] | ~1 [saline] | No data | No data | [15] |
| [99mTc]Tc-citrate (SnCl2) | At origin [acetone] | ~1 [saline] | No data | −2.46 * | [17] |
| No data | No data | [18] | |||
| [99mTc]Tc-AA (SnCl2) | 0.0–0.15 [serum physiologic] | 0.8–1.0 [ACD] ** | 1.04 *** | No data | [21] |
| [99mTc]Tc-AA (FeCl3) | No data | No data | 3.05 **** | No data | [22] |
| [99mTc]Tc-α-hydroxy acids (SnCl2) | At origin [acetone] | ~1 [saline] | No data | No data | [23] |
| [99mTc]Tc-gluconate (SnCl2) | At origin [acetone] | ~1 [saline] | No data | No data | [24] |
| Potassium Tartrate [mg] | RCY [%] |
|---|---|
| 0.22 | 15 |
| 0.44 | 60 |
| 0.66 | 88 |
| 0.88 | 90 |
| 1.1 | >95 |
| 1.32 | >95 |
| Potassium Tartrate [mg] | MTX [mg] | RCY in 5 min [%] | RCY in 120 min [%] |
|---|---|---|---|
| 1.1 | 1 | 25 | 59 |
| 1.1 | 2.5 | 27 | 72 |
| 1.1 | 5 | 41 | 92 |
| 1.1 | 10 | 44 | 87 |
| 99mTc-Radiocomplex | PC Rf Values | HPLC RT [min] | logP | |
|---|---|---|---|---|
| Acetone | Saline | |||
| [99mTc]Tc-AA | ~0 | ~1 | 5.8 | −3.08 ± 0.09 |
| [99mTc]Tc-EDTA | ~0 | ~1 | 5.4 | −3.99 ± 0.11 |
| [99mTc]Tc-gluconate | ~0 | ~1 | 5.1 | −4.05 ± 0.07 |
| [99mTc]Tc-tartrate | ~0 | ~1 | 5.6 | −2.46 ± 0.03 |
| [99mTc]Tc-MTX (not isolated) | ~0 | ~1 | 9.1 | −2.08 ± 0.05 * |
| [99mTc]Tc-MTX (isolated) | ~0 | ~1 | 9.1 | −1.54 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Common Shortcomings in Study on Radiopharmaceutical Design Research: A Case Study of 99mTc-Labelled Methotrexate. Molecules 2021, 26, 5862. https://doi.org/10.3390/molecules26195862
Koźmiński P, Halik PK, Chesori R, Gniazdowska E. Common Shortcomings in Study on Radiopharmaceutical Design Research: A Case Study of 99mTc-Labelled Methotrexate. Molecules. 2021; 26(19):5862. https://doi.org/10.3390/molecules26195862
Chicago/Turabian StyleKoźmiński, Przemysław, Paweł Krzysztof Halik, Raphael Chesori, and Ewa Gniazdowska. 2021. "Common Shortcomings in Study on Radiopharmaceutical Design Research: A Case Study of 99mTc-Labelled Methotrexate" Molecules 26, no. 19: 5862. https://doi.org/10.3390/molecules26195862