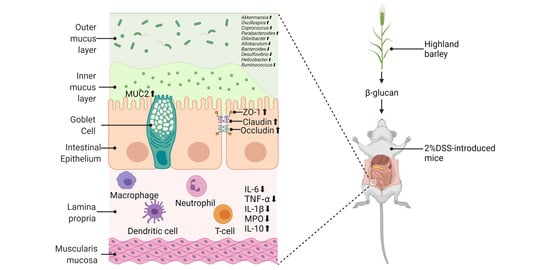
β-Glucan Extracted from Highland Barley Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice
Abstract
:1. Introduction
2. Results
2.1. The Effects of HBBG on Histopathological Indicators
2.2. The Effects of HBBG on the Intestinal Barrier
2.3. The Effects of HBBG on Concentration of MPO
2.4. The Effects of HBBG on Serum Inflammatory Cytokines
2.5. The Effects of HBBG on Intestinal Flora
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. MICE and Experimental Design
4.3. Assessment of DAI
4.4. Histopathological Analysis
4.5. Intestinal Permeability Test
4.6. RNA Isolation and RT-qPCR
4.7. Determination of the Content of MPO in Colon Tissue
4.8. The Level of Cytokines in Serum
4.9. Analysis of Intestinal Flora
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-Z. Inflammatory Bowel Disease: Pathogenesis. WJG 2014, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Ahuja, V.; Kedia, S.; Midha, V.; Mahajan, R.; Mehta, V.; Sudhakar, R.; Singh, A.; Kumar, A.; Puri, A.S.; et al. Diet and Inflammatory Bowel Disease: The Asian Working Group Guidelines. Indian J Gastroenterol. 2019, 38, 220–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from Natural Resources Exhibit Great Potential in the Treatment of Ulcerative Colitis: A Review. Carbohydrate Polymers 2021, 254, 117189. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Williams, B.; Grant, L.; Gidley, M.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. IJMS 2017, 18, 2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-Modulatory Effects of Dietary Yeast Beta-1,3/1,6-D-Glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.D.; Williams, D.L. Chapter 4.5.2—(1,3)-β-Glucans in Innate Immunity: Mammalian Systems. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 579–619. [Google Scholar] [CrossRef]
- Han, F.; Fan, H.; Yao, M.; Yang, S.; Han, J. Oral Administration of Yeast β-Glucan Ameliorates Inflammation and Intestinal Barrier in Dextran Sodium Sulfate-Induced Acute Colitis. J. Funct. Foods 2017, 35, 115–126. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Q.; Yang, T.; Nie, Y.; Li, X.; Liu, B.; Shen, J.; Liang, Y.; Tang, Y.; Luo, F. Oral Administration of Lentinus Edodes β-Glucans Ameliorates DSS-Induced Ulcerative Colitis in Mice via MAPK-Elk-1 and MAPK-PPARγ Pathways. Food Funct. 2016, 7, 4614–4627. [Google Scholar] [CrossRef]
- Liu, B.; Lin, Q.; Yang, T.; Zeng, L.; Shi, L.; Chen, Y.; Luo, F. Oat β-Glucan Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis in Mice. Food Funct. 2015, 6, 3454–3463. [Google Scholar] [CrossRef]
- Gong, L.; Gong, L.; Zhang, Y. Intake of Tibetan Hull-Less Barley Is Associated with a Reduced Risk of Metabolic Related Syndrome in Rats Fed High-Fat-Sucrose Diets. Nutrients 2014, 6, 1635–1648. [Google Scholar] [CrossRef]
- Charles, M.T.; Mercier, J.; Makhlouf, J.; Arul, J. Physiological Basis of UV-C-Induced Resistance to Botrytis Cinerea in Tomato Fruit. Postharvest Biol. Technol. 2008, 47, 10–20. [Google Scholar] [CrossRef]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the Nutrient Composition and Nutritional Functions of Highland Barley (Qingke): A Review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Zhang, G.; Junmei, W.; Jinxin, C. Analysis of B-Glucan Content in Barley Cultivars from Different Locations of China. Food Chem. 2002, 79, 251–254. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Starch Digestibility and Bioactivity of High Altitude Hulless Barley. Food Chem. 2016, 194, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, G.; Xing, Y.; Ding, Y.; Ren, T.; Kan, J. Antioxidant Activity of Whole Grain Highland Hull-Less Barley and Its Effect on Liver Protein Expression Profiles in Rats Fed with High-Fat Diets. Eur. J. Nutr. 2018, 57, 2201–2208. [Google Scholar] [CrossRef]
- Tang, T.; Song, J.; Wang, H.; Zhang, Y.; Xin, J.; Suo, H. Qingke Beta-Glucan Synergizes with a Beta-Glucan-Utilizing Lactobacillus Strain to Relieve Capsaicin-Induced Gastrointestinal Injury in Mice. Int. J. Biol. Macromol. 2021, 174, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nie, Q.; Xie, M.; Yao, H.; Zhang, K.; Yin, J.; Nie, S. Protective Effects of β-Glucan Isolated from Highland Barley on Ethanol-Induced Gastric Damage in Rats and Its Benefits to Mice Gut Conditions. Food Res. Int. 2019, 122, 157–166. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A Novel Method in the Induction of Reliable Experimental Acute and Chronic Ulcerative Colitis in Mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative Assay for Acute Intestinal Inflammation Based on Myeloperoxidase Activity. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The Inner of the Two Muc2 Mucin-Dependent Mucus Layers in Colon Is Devoid of Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [Green Version]
- Heazlewood, C.K.; Cook, M.C.; Eri, R.; Price, G.R.; Tauro, S.B.; Taupin, D.; Thornton, D.J.; Png, C.W.; Crockford, T.L.; Cornall, R.J.; et al. Aberrant Mucin Assembly in Mice Causes Endoplasmic Reticulum Stress and Spontaneous Inflammation Resembling Ulcerative Colitis. PLoS Med. 2008, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-Induced Increase in Intestinal Epithelial Tight Junction Permeability Requires NF-κB Activation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sadi, R.M.; Ma, T.Y. IL-1β Causes an Increase in Intestinal Epithelial Tight Junction Permeability. J. Immunol. 2007, 178, 4641–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigmann, B.; Lehr, H.A.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; Stevens, S.; Schmidt, J.; Galle, P.R.; Rose-John, S.; Neurath, M.F. The Transcription Factor NFATc2 Controls IL-6–Dependent T Cell Activation in Experimental Colitis. J. Exp. Med. 2008, 205, 2099–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, P.; Peng, Y.; Chen, G.; Xie, M.; Dai, Z.; Huang, K.; Dong, W.; Zeng, X.; Sun, Y. Modulation of Gut Microbiota by Ilex Kudingcha Improves Dextran Sulfate Sodium-Induced Colitis. Food Res. Int. 2019, 126, 108595. [Google Scholar] [CrossRef]
- Madsen, K.; Lewis, S.; Tavernini, M.; Hibbard, J.; Fedorak, R. Interleukin 10 Prevents Cytokine-Induced Disruption of T84 Monolayer Barrier Integrity and Limits Chloride Secretion. Gastroenterology 1997, 113, 151–159. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Spor, A.; Koren, O.; Ley, R. Unravelling the Effects of the Environment and Host Genotype on the Gut Microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Perry, S.; de la Luz Sanchez, M.; Yang, S.; Haggerty, T.; Hurst, P.; Perez-Perez, G.; Parsonnet, J. Gastroenteritis and Transmission of Helicobacter Pylori Infection in Households. Emerg. Infect. Dis. 2006, 12, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.J.; Ahmed, Y.M.; Zamzami, M.A.; Mohamed, S.A.; Khan, I.; Baothman, O.A.S.; Mehanna, M.G.; Yasir, M. Effect of Atorvastatin on the Gut Microbiota of High Fat Diet-Induced Hypercholesterolemic Rats. Sci. Rep. 2018, 8, 662. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia Muciniphila gen. nov., sp. nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Passel, M.W.J.; Kant, R.; Zoetendal, E.G.; Plugge, C.M.; Derrien, M.; Malfatti, S.A.; Chain, P.S.G.; Woyke, T.; Palva, A.; de Vos, W.M.; et al. The Genome of Akkermansia Muciniphila, a Dedicated Intestinal Mucin Degrader, and Its Use in Exploring Intestinal Metagenomes. PLoS ONE 2011, 6, e16876. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.-M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-Clinical Detection of Gut Microbial Biomarkers of Obesity and Type 2 Diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8 + T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, F.; Wang, W.; Sun, C.; Gao, D.; Ma, J.; Hussain, M.A.; Xu, C.; Jiang, Z.; Hou, J. Study of the Alleviation Effects of a Combination of Lactobacillus Rhamnosus and Inulin on Mice with Colitis. Food Funct. 2020, 11, 3823–3837. [Google Scholar] [CrossRef]
- Silvestri, C.; Pagano, E.; Lacroix, S.; Venneri, T.; Cristiano, C.; Calignano, A.; Parisi, O.A.; Izzo, A.A.; Di Marzo, V.; Borrelli, F. Fish Oil, Cannabidiol and the Gut Microbiota: An Investigation in a Murine Model of Colitis. Front. Pharmacol. 2020, 11, 585096. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of Prebiotic-Treated Mice Reveals Novel Targets Involved in Host Response during Obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E. The Composition of the Gut Microbiota Shapes the Colon Mucus Barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Gut Microbiota: Effect of Pubertal Status. BMC Microbiol. 2020, 20, 334. [Google Scholar] [CrossRef]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, Inflammation, and Response to Treatment: A Longitudinal Study of Pediatric Subjects with Newly Diagnosed Inflammatory Bowel Disease. Genome Med. 2016, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, T.W.; Pham, H.-P.; Bridonneau, C.; Aubry, C.; Lamas, B.; Martin-Gallausiaux, C.; Moroldo, M.; Rainteau, D.; Lapaque, N.; Six, A.; et al. Microorganisms Linked to Inflammatory Bowel Disease-Associated Dysbiosis Differentially Impact Host Physiology in Gnotobiotic Mice. ISME J. 2016, 10, 460–477. [Google Scholar] [CrossRef] [Green Version]
- Lehours, P.; Ferrero, R.L. Review: Helicobacter: Inflammation, Immunology, and Vaccines. Helicobacter 2019, 24, e12737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nono, I.; Ohno, N.; Masuda, A.; Oikawa, S.; Yadomae, T. Oxidative Degradation of an Antitumor (1-3)-β-D-Glucan, Grifolan. J. Pharm.-Dyn. 1991, 14, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dongowski, G.; Huth, M.; Gebhardt, E.; Flamme, W. Dietary Fiber-Rich Barley Products Beneficially Affect the Intestinal Tract of Rats. J. Nutr. 2002, 132, 3704–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulcher, G.; Slavin, J.L. Concentrated Oat β-Glucan, a Fermentable Fiber, Lowers Serum Cholesterol in Hypercholesterolemic Adults in a Randomized Controlled Trial. Nutr. J. 2007, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermudez-Brito, M.; Sahasrabudhe, N.M.; Faas, M.M.; de Vos, P. The Impact of Dietary Fibers on Dendritic Cell Responses in Vitro Is Dependent on the Differential Effects of the Fibers on Intestinal Epithelial Cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; de Vos, P. Toll-Like Receptor 2 Activation by Β2→1-Fructans Protects Barrier Function of T84 Human Intestinal Epithelial Cells in a Chain Length–Dependent Manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Westerlund, E.; Andersson, R.; Åman, P. Isolation and Chemical Characterization of Water-Soluble Mixed-Linked β-Glucans and Arabinoxylans in Oat Milling Fractions. Carbohydr. Polym. 1993, 20, 115–123. [Google Scholar] [CrossRef]
- Murthy, S.N.S.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of Dextran Sulfate Sodium-Induced Murine Colitis by Intracolonic Cyclosporin. Digest. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef]
- Steedman, H.F. Alcian Blue 8GS: A New Stain for Mucin. Q. J. Microsc. Sci. 1950, 91, 477–479. [Google Scholar]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Fang, C.H.; Hasselgren, P.-O. Intestinal Permeability Is Reduced and IL-10 Levels Are Increased in Septic IL-6 Knockout Mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, R1013–R1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yan, W.; Sun, C.; Ji, C.; Zhou, Q.; Zhang, D.; Zheng, J.; Yang, N. The Gut Microbiota Is Largely Independent of Host Genetics in Regulating Fat Deposition in Chickens. ISME J. 2019, 13, 1422–1436. [Google Scholar] [CrossRef] [PubMed]







| Weight Loss | Stool Consistency | Occult Blood or Gross Bleeding | Score |
|---|---|---|---|
| 0 | Normal | negative (−) | 0 |
| 1–5 | Loose stools | positive (+) | 1 |
| 5–10 | Loose stools | positive (++) | 2 |
| 10–15 | Diarrhea | positive (+++) | 3 |
| >15 | Diarrhea | Gross bleeding | 4 |
| Crypt Damage | Range of Lesion (%) | Degree of Inflammatory Infiltration | Depth of Lesions | Score |
|---|---|---|---|---|
| None | 0% | None | None | 0 |
| Loss of bottom one-third of the crypt | 1–25% | Mild | Mucosal layer | 1 |
| Loss of bottom two-thirds of the crypt | 26–50% | Moderate | Submucosa | 2 |
| Loss of entire crypt with the surface epithelium remaining intact | 51–75% | Severe | Muscular layer and serosal layer | 3 |
| Loss of the entire crypt and surface epithelium | 76–100% | Severe | Muscular layer and serosal layer | 4 |
| Gene | Upstream Primer (5′–3′) | Downstream Primer (5′–3′) |
|---|---|---|
| claudin-1 | CGGGCAGATACAGTGCAAAG | ACTTCATGCCAATGGTGGAC |
| occludin | ATGTCCGGCCGATGCTCTC | TTTGGCTGCTCTTGGGTCTGTA |
| ZO-1 | TTTTTGACAGGGGGAGTGG | TGCTGCAGAGGTCAAAGTTCAAG |
| MUC2 | ATGCCCACCTCCTCAAAGAC | GTAGTTTCCGTTGGAACAGTGAA |
| GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Tian, S.; Li, S.; Pang, X.; Sun, J.; Zhu, X.; Lv, F.; Lu, Z.; Li, X. β-Glucan Extracted from Highland Barley Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice. Molecules 2021, 26, 5812. https://doi.org/10.3390/molecules26195812
Chen M, Tian S, Li S, Pang X, Sun J, Zhu X, Lv F, Lu Z, Li X. β-Glucan Extracted from Highland Barley Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice. Molecules. 2021; 26(19):5812. https://doi.org/10.3390/molecules26195812
Chicago/Turabian StyleChen, Minjie, Shuhua Tian, Shichao Li, Xinyi Pang, Jing Sun, Xiaoyu Zhu, Fengxia Lv, Zhaoxin Lu, and Xiangfei Li. 2021. "β-Glucan Extracted from Highland Barley Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice" Molecules 26, no. 19: 5812. https://doi.org/10.3390/molecules26195812