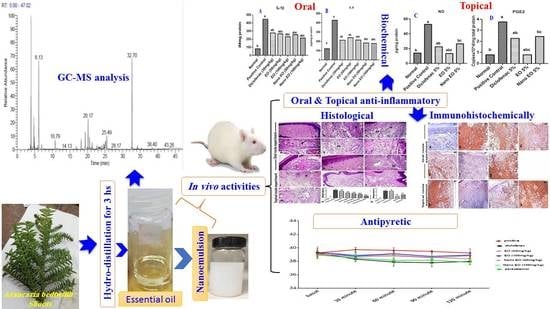
Oral and Topical Anti-Inflammatory and Antipyretic Potentialities of Araucaria bidiwillii Shoot Essential Oil and Its Nanoemulsion in Relation to Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Drugs and Chemicals
2.3. Essential Oil Extraction, GC–MS Analysis and Chemical Constituents’ Identification
2.4. ABSEO Nanoemulsion Preparation
2.5. Droplet Size and Zeta Potential Analysis
2.6. Biological Experimental Section
2.6.1. Experimental Animals and Ethical Statements
2.6.2. Evaluation of Acute Toxicity
2.6.3. Anti-Inflammatory and Antipyretic Effects of Oral Administration of ABSEO and Nanoemulsion
Carrageenan-Induced Rat Paw Edema
2.6.4. Antipyretic Effect
2.6.5. Topical Anti-Inflammatory Effect of ABSEO and Nanoemulsion
Carrageenan-Induced Rat Paw Edema
2.6.6. Homogenate Preparation
2.6.7. Assessment of Inflammatory Cytokines and Nitrosative Biomarker
2.6.8. PGE2 Gene Expression Analysis
2.6.9. Hematoxylin and Eosin (H&E) Staining and Inflammation Scoring
2.6.10. Immunohistochemical Examination
2.6.11. Statistical Method
3. Results
3.1. Chemical Profile of ABSEO
3.2. Essential Oil Nanoemulsion
3.3. Results of Oral Anti-Inflammatory Effect of ABSEO and Its Anoemulsion
3.3.1. Effect on Interleukin 1β (IL-1β) and 8 (IL-8)
3.3.2. Effect of Nitric Oxide (NO)
3.3.3. Quantification of Prostaglandin E2 (PGE2) RNA Expression
3.4. Result of Antipyretic Effect
3.5. Results of Topical Anti-Inflammatory Effect of ABSEO and Its Nanoemulsion
3.5.1. Carrageenan-Induced Rat Paw Edema
3.5.2. Effect on Interleukin 1β (IL-1β), and 8 (IL-8)
3.5.3. Effect of Nitrosative Biomarker (Nitric Oxide; NO)
3.5.4. Quantification of Prostaglandin E2 (PGE2) RNA Expression
3.6. Histopathological Findings
3.7. Immunohistochemistry Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Elkady, W.M.; Ayoub, I.M. Chemical Profiling and Antiproliferative Effect of Essential Oils of Two Araucaria Species Cultivated in Egypt. Ind. Crop. Prod. 2018, 118, 188–195. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical Profile of Launaea nudicaulis Ethanolic Extract and Its Antidiabetic Effect in Streptozotocin-Induced Rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef]
- Saleh, I.; Abd-ElGawad, A.; el Gendy, A.E.-N.; Abd El Aty, A.; Mohamed, T.; Kassem, H.; Aldosri, F.; Elshamy, A.; Hegazy, M.-E.F. Phytotoxic and Antimicrobial Activities of Teucrium Polium and Thymus Decussatus Essential Oils Extracted Using Hydrodistillation and Microwave-Assisted Techniques. Plants 2020, 9, 716. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-κB Signaling Pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Ammar, N.M.; Abdelhameed, M.F.; el Gendy, A.E.-N.G.; Ragab, T.I.M.; Abd-Elgawad, A.M.; Farag, M.A.; Alwahibi, M.S.; Elshamy, A.I. Protective Mechanism of Acacia Saligna Butanol Extract and Its Nano-Formulations against Ulcerative Colitis in Rats as Revealed via Biochemical and Metabolomic Assays. Biology 2020, 9, 195. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Hammam, W.E.; El-Mahdy El-Tantawi, M.; Yassin, N.A.Z.; Kirollos, F.N.; Abdelhameed, M.F.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. Apple Leaves and Their Major Secondary Metabolite Phlorizin Exhibit Distinct Neuroprotective Activities: Evidence from in Vivo and in Silico Studies. Arab. J. Chem. 2021, 14, 103188. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Karim, S.S.; Mohamed, H.S.; Abdelhameed, M.F.; El-Galil E Amr, A.; Almehizia, A.A.; Nossier, E.S. Design, Synthesis and Molecular Docking of New Pyrazole-Thiazolidinones as Potent Anti-Inflammatory and Analgesic Agents with TNF-α Inhibitory Activity. Bioorganic Chem. 2021, 111, 104827. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Ndisang, J.F. Role of Heme Oxygenase in Inflammation, Insulin-Signalling, Diabetes and Obesity. Mediat. Inflamm. 2010, 2010, 359732. [Google Scholar] [CrossRef] [Green Version]
- Bashandy, S.A.E.; Salama, A.; Fayed, A.M.; Omara, E.A.; El-Toumy, S.A.; Salib, J.Y. Protective Effect of Mandrin (Citrus reticulata) Peel Extract on Potassium Dichromate Induced Hepatotoxicity and Nephrotoxicity in Rats. Plant Arch. 2020, 20, 2231–2242. [Google Scholar]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-Antioxidant Signaling Attenuates NFκB-Inflammatory Response and Elicits Apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, A.M.; Almeida, M.T.R.; Andrighetti-Fröhner, C.R.; Cardozo, F.; Barardi, C.R.M.; Farias, M.R.; Simões, C.M.O. Antiviral Activity-Guided Fractionation from Araucaria angustifolia Leaves Extract. J. Ethnopharmacol. 2009, 126, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.M.M.; Lima, S.R.; Soares, P.M.; Assreuy, A.M.S.; de Sousa, F.C.F.; Lobato, R.d.F.G.; Vasconcelos, G.S.; Santi-Gadelha, T.; Bezerra, E.H.S.; Cavada, B.S. Central Action of Araucaria angustifolia Seed Lectin in Mice. Epilepsy Behav. 2009, 15, 291–293. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Monem, A.R.A.; Ezzat, S.M.; El-Halawany, A.M.; Mouneir, S.M. Chemical and Biological Investigation of Araucaria heterophylla Salisb. Resin. Z. Nat. C 2009, 64, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.N.; Awad, H.M.; El-Sayed, N.H.; Paré, P.W. Chemical and Antioxidant Investigations: Norfolk Pine Needles (Araucaria excelsa). Pharm. Biol. 2010, 48, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Goldsack, R.J.; Wu, M.Z.; Fookes, C.J.R.; Forster, P.I. The Steam Volatile Oil of Wollemia nobilis and Its Comparison with Other Members of the Araucariaceae (Agathis and Araucaria). Biochem. Syst. Ecol. 2000, 28, 563–578. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; el Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential Oil and Its Nanoemulsion of Araucaria heterophylla Resin: Chemical Characterization, Anti-Inflammatory, and Antipyretic Activities. Ind. Crop. Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Goswami, P.; Verma, S.K.; Chauhan, A.; Darokar, M.P. Chemical Composition and Antibacterial Activity of Foliage and Resin Essential Oils of Araucaria cunninghamii Aiton Ex D. Don and Araucaria heterophylla (Salisb.) Franco from India. Ind. Crop. Prod. 2014, 61, 410–416. [Google Scholar] [CrossRef]
- Dold, A.P.; Cocks, M.L. The Medicinal Use of Some Weeds, Problem and Alien Plants in the Grahamstown and Peddie Districts of the Eastern Cape, South Africa. S. Afr. J. Sci. 2000, 96, 467–474. [Google Scholar]
- El-Hawary, S.S.; Rabeh, M.A.; el Raey, M.A.; El-Kadder, E.M.A.; Sobeh, M.; Abdelmohsen, U.R.; Albohy, A.; Andrianov, A.M.; Bosko, I.P.; Al-Sanea, M.M.; et al. Metabolomic Profiling of Three Araucaria species, and Their Possible Potential Role against COVID-19. J. Biomol. Struct. Dyn. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abdelhameed, M.F.; Mostafa, S.; Nada, S.A.; Taha, H.S.; Amer, A.A. Influence of Extract Derived Cell Cultures of Broccoli against Osteoporosis in Ovariectomized Rats. Egypt. J. Chem. 2021, 64, 3521–3539. [Google Scholar] [CrossRef]
- Essa, A.F.; El-Hawary, S.S.; Abd-El Gawad, A.M.; Kubacy, T.M.; El-Khrisy, E.E.A.M.; Elshamy, A.I.; Younis, I.Y. Prevalence of Diterpenes in Essential Oil of Euphorbia mauritanica L.: Detailed Chemical Profile, Antioxidant, Cytotoxic and Phytotoxic Activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Afia, S.I.; Mohafrash, S.M.M.; Abou-Awad, B.A. Formulation and Characterization of Garlic (Allium sativum L.) Essential Oil Nanoemulsion and Its Acaricidal Activity on Eriophyid Olive Mites (Acari: Eriophyidae). Environ. Sci. Pollut. Res. 2018, 25, 10526–10537. [Google Scholar] [CrossRef]
- Fernandes, C.P.; de Almeida, F.B.; Silveira, A.N.; Gonzalez, M.S.; Mello, C.B.; Feder, D.; Apolinário, R.; Santos, M.G.; Carvalho, J.C.T.; Tietbohl, L.A.C. Development of an Insecticidal Nanoemulsion with Manilkara subsericea (Sapotaceae) Extract. J. Nanobiotechnol. 2014, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-Inflammatory Effect and Potential Mechanism of Betulinic Acid on λ-Carrageenan-Induced Paw Edema in Mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef]
- Khalifa, N.M.; Al-Omar, M.A.; Amr, A.E.-G.E.; Baiuomy, A.R.; Abdel-Rahman, R.F. Synthesis and Biological Evaluation of Some Novel Fused Thiazolo [3,2-a]Pyrimidines as Potential Analgesic and Anti-Inflammatory Agents. Russ. J. Bioorganic Chem. 2015, 41, 192–200. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Asaad, G.F.; Arbid, M.S.; Abdel-Sattar, E.A. Anti-Inflammatory, Antinociceptive, Antipyretic and Gastroprotective Effects of Calligonum comosum in Rats and Mice. Int. J. Toxicol. Pharmacol. Res. 2014, 6, 26–33. [Google Scholar]
- Saini, N.K.; Singha, M. Anti-Inflammatory, Analgesic and Antipyretic Activity of Methanolic Tecomaria capensis Leaves Extract. Asian Pac. J. Trop. Biomed. 2012, 2, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Roszkowski, A.P.; Rooks, W.H.; Tomolonis, A.J.; Miller, L.M. Anti-Inflammatory and Analgetic Properties of d-2-(6′-Methoxy-2′-Naphthyl)-Propionic Acid (Naproxen). J. Pharmacol. Exp. Ther. 1971, 179, 114–123. [Google Scholar] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: North Hollywood, CA, USA, 2008; ISBN 0443102791. [Google Scholar]
- Emam, M.; Moustafa, P.E.; Elkhateeb, A.; Hussein, S.R.; Marzouk, M.M.; Abd El-Rahman, S.S.; Abdel-Hameed, E.S.S.; Abdel-Rahman, R.F. Dobera glabra (Forssk.) Poir. (Salvadoraceae); Phenolic Constituents of the Aqueous Leaves Extract and Evaluation of Its Anti-Inflammatory, Analgesic Activities. Heliyon 2021, 7, e06205. [Google Scholar] [CrossRef]
- Hsu, S.M.; Raine, L.; Fanger, H. The Use of Antiavidin Antibody and Avidin-Biotin-Peroxidase Complex in Immunoperoxidase Technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; el Gendy, A.E.G.; Al-Rowaily, S.L. Interspecific Variation, Antioxidant and Allelopathic Activity of the Essential Oil from Three Launaea Species Growing Naturally in Heterogeneous Habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; el Gendy, A.E.-N.G.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential Oil of Calotropis procera: Comparative Chemical Profiles, Antimicrobial Activity, and Allelopathic Potential on Weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef]
- Pietsch, M.; König, W.A. Enantiomers of Sesquiterpene and Diterpene Hydrocarbons in Araucaria Species. Phytochem. Anal. 2000, 11, 99–105. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; el Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Omer, E.A.; Dar, B.A.; Al-Taisan, W.A.; Elshamy, A.I. Essential Oil Enriched with Oxygenated Constituents from Invasive Plant Argemone ochroleuca Exhibited Potent Phytotoxic Effects. Plants 2020, 9, 998. [Google Scholar] [CrossRef]
- Araújo, F.A.; Kelmann, R.G.; Araújo, B.V.; Finatto, R.B.; Teixeira, H.F.; Koester, L.S. Development and Characterization of Parenteral Nanoemulsions Containing Thalidomide. Eur. J. Pharm. Sci. 2011, 42, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Gill, R.; Chen, M.L.; Zhang, F.; Linhardt, R.J.; Dudeja, P.K.; Tobacman, J.K. Toll-like Receptor 4 Mediates Induction of the Bcl10-NFkappaB-Interleukin-8 Inflammatory Pathway by Carrageenan in Human Intestinal Epithelial Cells. J. Biol. Chem. 2008, 283, 10550–10558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.; Jung, Y.; Choi, Y.; Li, S. Effects of Er-Miao-San Extracts on TNF-Alpha-Induced MMP-1 Expression in Human Dermal Fibroblasts. Biol. Res. 2015, 48, 8. [Google Scholar] [CrossRef] [Green Version]
- Dangarembizi, R.; Erlwanger, K.H.; Rummel, C.; Roth, J.; Madziva, M.T.; Harden, L.M. Brewer’s Yeast Is a Potent Inducer of Fever, Sickness Behavior and Inflammation within the Brain. Brain Behav. Immun. 2018, 68, 211–223. [Google Scholar] [CrossRef]
- Larsson, K.; Kock, A.; Idborg, H.; Arsenian Henriksson, M.; Martinsson, T.; Johnsen, J.I.; Korotkova, M.; Kogner, P.; Jakobsson, P.-J. COX/MPGES-1/PGE2 Pathway Depicts an Inflammatory-Dependent High-Risk Neuroblastoma Subset. Proc. Natl. Acad. Sci. USA 2015, 112, 8070–8075. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Kiritsakis, A.K.; Kiritsakis, K.A.; Tsitsipas, C.K. A Review of the Evolution in the Research of Antioxidants in Olives and Olive Oil during the Last Four Decades. J. Food Bioact. 2020, 11, 31–56. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive Oil Phenolics Are Dose-Dependently Absorbed in Humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Borah, A.; Paw, M.; Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Pandey, S.K.; Lal, M. Chemical Composition, Antioxidant, Anti-Inflammatory, Anti-Microbial and in-Vitro Cytotoxic Efficacy of Essential Oil of Curcuma caesia Roxb. Leaves: An Endangered Medicinal Plant of North East India. Ind. Crop. Prod. 2019, 129, 448–454. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-Inflammatory Activity of Clove (Eugenia caryophyllata) Essential Oil in Human Dermal Fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumar, R.; Prakash, O.; Srivastava, R.M.; Pant, A.K. Chemical Composition, in vitro Antioxidant, Anti-Inflammatory and Antifeedant Properties in the Essential Oil of Asian Marsh Weed Limnophila indica L.(Druce). J. Pharmacogn. Phytochem. 2019, 8, 1689–1694. [Google Scholar]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Ribeiro-Filho, J.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Quintans Júnior, L.J.; Araújo, A.A.d.S.; et al. Anti-Inflammatory Activity of the Essential Oil Obtained from Ocimum basilicum Complexed with β-Cyclodextrin (β-CD) in Mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109, 836–846. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical Composition, Anti-Inflammatory Activity and Cytotoxic Effects of Essential Oils from Three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, W.; Kim, S.; Oh, T.; Lee, N.H.; Hyun, C. Abies koreana Essential Oil Inhibits Drug-resistant Skin Pathogen Growth and LPS-induced Inflammatory Effects of Murine Macrophage. Lipids 2009, 44, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, H.-J.; Jeon, Y.-D.; Han, Y.-H.; Kee, J.-Y.; Kim, H.-J.; Shin, H.-J.; Kang, J.; Lee, B.S.; Kim, S.-H. Alpha-Pinene Exhibits Anti-Inflammatory Activity through the Suppression of MAPKs and the NF-ΚB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-Inflammatory and Chondroprotective Activity of (+)-α-Pinene: Structural and Enantiomeric Selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Özbek, H.; Yılmaz, B.S. Anti-Inflammatory and Hypoglycemic Activities of Alpha-Pinene. ACTA Pharm. Sci. 2017, 55, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. Β-caryophyllene and Β-caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; da Silva, A.J.R.; Dias Fernandes, P. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e0153643. [Google Scholar]
- Del-Vechio-Vieira, G.; de Sousa, O.V.; Miranda, M.A.; Senna-Valle, L.; Kaplan, M.A.C. Analgesic and Anti-Inflammatory Properties of Essential Oil from Ageratum fastigiatum. Braz. Arch. Biol. Technol. 2009, 52, 1115–1121. [Google Scholar] [CrossRef]
- van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid.-Based Complementary Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| No | Current Study | % of Combined Compounds in Previous Studies | |||||
|---|---|---|---|---|---|---|---|
| Rt a | Rel. Conc. % b | Component Name c | M.F. d | KI e | Egyptian Foliage f | Australian Leaf g | |
| Monoterpene Hydrocarbons | |||||||
| 1 | 3.72 | 0.85 ± 0.02 | Tricyclene | C10H16 | 921 | --- | --- |
| 2 | 3.92 | 16.21 ± 0.78 | α-Pinene | C10H16 | 932 | 1.52 | 0.5 |
| 3 | 4.28 | 0.38 ± 0.02 | Camphene | C10H16 | 946 | --- | --- |
| 4 | 4.74 | 4.12 ± 0.11 | Sabinene | C10H16 | 969 | --- | --- |
| 5 | 4.87 | 1.51 ± 0.05 | β-Pinene | C10H16 | 979 | 0.02 | traces |
| 6 | 5.11 | 0.41 ± 0.01 | α-Myrcene | C10H16 | 990 | 0.03 | traces |
| 7 | 5.49 | 0.16 ± 0.01 | α-Phellandrene | C10H16 | 1002 | --- | --- |
| 8 | 5.56 | 0.25 ± 0.02 | Pseudolimonene | C10H16 | 1004 | --- | --- |
| 9 | 5.81 | 0.69 ± 0.04 | α-Terpinene | C10H16 | 1014 | --- | --- |
| 10 | 6.13 | 14.22 ± 0.16 | D-Limonene | C10H16 | 1024 | 0.11 | 0.1 |
| 11 | 6.94 | 1.03 ± 0.04 | γ-Terpinene | C10H16 | 1054 | 0.02 | --- |
| 12 | 7.72 | 0.21 ± 0.02 | α-Terpinolene | C10H16 | 1086 | --- | --- |
| Monoterpene Alcohols | |||||||
| 13 | 9.52 | 0.17 ± 0.02 | trans-Pinocarveol | C10H16O | 1139 | --- | --- |
| 14 | 10.54 | 0.11 ± 0.01 | Borneol | C10H18O | 1165 | --- | --- |
| 15 | 10.78 | 2.21 ± 0.06 | δ-Terpineol | C10H18O | 1166 | --- | --- |
| 16 | 11.39 | 0.32 ± 0.03 | 4-Terpineol | C10H18O | 1174 | --- | --- |
| 17 | 16.12 | 0.09 ± 0.01 | Terpinen-4-yl acetate | C12H20O2 | 1299 | --- | --- |
| Sesquiterpene Hydrocarbons | |||||||
| 18 | 16.8 | 0.71 ± 0.04 | α-Copaene | C15H24 | 1375 | 0.20 | traces |
| 19 | 17.27 | 0.13 ± 0.01 | β-Elemene | C15H24 | 1389 | 0.20 | --- |
| 20 | 18.2 | 2.16 ± 0.10 | β-Caryophyllene | C15H24 | 1408 | 0.19 | 0.1 |
| 21 | 19.36 | 4.14 ± 0.13 | β-Humulene | C15H24 | 1436 | --- | 0.1 |
| 22 | 19.98 | 0.43 ± 0.02 | γ-Muurolene | C15H24 | 1478 | 0.79 | --- |
| 23 | 20.17 | 6.69 ± 0.21 | Germacrene D | C15H24 | 1484 | 5.53 | 0.2 |
| 24 | 20.44 | 0.10 ± 0.01 | β-Selinene | C15H24 | 1489 | --- | --- |
| 25 | 20.6 | 2.31 ± 0.08 | Bicyclogermacrene | C15H24 | 1500 | --- | 0.2 |
| 26 | 20.74 | 0.22 ± 0.01 | α-Muurolene | C15H24 | 1500 | --- | --- |
| 27 | 21.19 | 0.40 ± 0.02 | δ-Amorphene | C15H24 | 1512 | --- | --- |
| 28 | 21.3 | 1.43 ± 0.04 | γ-Cadinene | C15H24 | 1513 | 2.03 | 0.1 |
| 29 | 22.15 | 0.10 ± 0.01 | α-Calacorene | C15H20 | 1544 | --- | --- |
| Sesquiterpene Alcohols | |||||||
| 30 | 22.71 | 1.01 ± 0.02 | trans-Nerolidol | C15H26O | 1531 | 13.66 | --- |
| 31 | 22.92 | 0.07 ± 0.01 | Spathulenol | C15H26O | 1577 | 1.09 | 3.2 |
| 32 | 23.17 | 0.20 ± 0.02 | Globulol | C15H24O | 1590 | --- | 0.2 |
| 33 | 23.66 | 0.64 ± 0.03 | Viridiflorol | C15H26O | 1600 | --- | --- |
| 34 | 23.95 | 0.56 ± 0.05 | Ledol | C15H26O | 1602 | --- | --- |
| 35 | 24.47 | 0.13 ± 0.01 | Fonenol | C15H26O | 1627 | --- | --- |
| 36 | 24.62 | 1.17 ± 0.07 | T-Cadinol | C15H26O | 1640 | 1.76 | --- |
| 37 | 25.1 | 3.49 ± 0.09 | T-Muurolol | C15H26O | 1642 | 2.51 | --- |
| 38 | 25.16 | 2.03 ± 0.08 | Cubenol | C15H26O | 1645 | --- | --- |
| 39 | 25.49 | 3.46 ± 0.12 | α-Cadinol | C15H26O | 1652 | --- | --- |
| 40 | 25.82 | 0.08 ± 0.01 | Khusinol | C15H24O | 1823 | --- | --- |
| Diterpene Hydrocarbons | |||||||
| 41 | 32.7 | 20.81 ± 0.21 | Beyerene | C20H32 | 1931 | 35.65 | --- |
| 42 | 33.41 | 1.07 ± 0.06 | Kaur-15-ene | C20H32 | 1997 | 1.37 | --- |
| Diterpene Alcohols | |||||||
| 43 | 34.22 | 1.86 ± 0.07 | Manool | C20H34O | 2056 | --- | --- |
| 40.04 | Monoterpene hydrocarbons | ||||||
| 2.90 | Monoterpene alcohols | ||||||
| 18.82 | Sesquiterpene hydrocarbons | ||||||
| 12.84 | Sesquiterpene alcohols | ||||||
| 21.88 | Diterpene hydrocarbons | ||||||
| 1.86 | Diterpene alcohols | ||||||
| 98.34 | Total | ||||||
| Groups | Baseline | 1 h | 2 h | 3 h | 4 h | ||||
|---|---|---|---|---|---|---|---|---|---|
| Paw Thickness (mm) | % Edema | % Inhibition | % Edema | % Inhibition | % Edema | % Inhibition | % Edema | % Inhibition | |
| Positive control | 2.39 ± 0.15 | 53.9 ± 9.37 | ------- | 72.2 ± 3.54 | ------- | 83.0 ± 5.25 | ------- | 76.8 ± 6.36 | ------- |
| Diclofenac (30 mg/kg) | 2.14 ± 0.1 | 46.7 ± 6.60 | 13.36 | 27.4 ± 7.7 | 62.04 | 18.5 ± 3.2 | 77.7 | 9.7 ± 1.3 | 76.7 |
| EO (50 mg/kg) | 2.14 ± 0.01 | 72.5 ± 10.7 | ------- | 73.8 ± 9.4 | ------- | 74.5 ± 8.4 | 10.24 | 69.2±14.3 | 9.89 |
| EO (100 mg/kg) | 2.27 ± 0.08 | 40.4 ± 7.3 | 25.04 | 82.6 ± 15.3 | ------- | 76.4 ± 18.2 | 7.95 | 73.5 ± 17.9 | 4.29 |
| Nano EO (50 mg/kg) | 2.29 ± 0.07 | 44.3 ± 8.2 | 17.8 | 68.6 ± 11.25 | 4.99 | 57.2 ± 13.6 | 31.08 | 40.3 ± 4.45 | 47.5 |
| Nano EO (100 mg/kg) | 2.39 ± 0.03 | 40.1 ± 8.7 | 25.6 | 62.6 ± 8.9 | 8.75 | 42.7 ± 6.9 | 48.55 | 32.1 ± 6.2 | 58.2 |
| Groups | Baseline | 1 h | 2 h | 3 h | 4 h | ||||
|---|---|---|---|---|---|---|---|---|---|
| Paw Thickness (mm) | % Edema | % Inhibition | % Edema | % Inhibition | % Edema | % Inhibition | % Edema | % Inhibition | |
| Positive control | 2.39 ± 0.15 | 53.9 ± 9.37 | ------- | 72.2 ± 3.54 | ------- | 83.0 ± 5.25 | ------- | 76.8 ± 6.36 | ------- |
| Diclofenac 5% | 2.15 ± 0.13 | 39.3 ± 8.86 | 27.09 | 95.3 ± 17.3 | ------- | 124.6 ± 19.8 | ------- | 95.8 ± 16.68 | ------- |
| EO 5% | 2.3 ± 0.2 | 36.4 ± 5.75 | 32.47 | 43 ± 19.64 | 40.44 | 32.6 ± 19.77 | 57.55 | 33.3 ± 11.49 | 59.87 |
| Nano EO 5% | 2.28 ± 0.07 | 26.4 ± 8.16 | 51.02 | 45.2 ± 8.64 | 37.39 | 67.2 ± 6.8 | 19.04 | 57.8 ± 1.01 | 24.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhameed, M.F.; Asaad, G.F.; Ragab, T.I.M.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd El-Rahman, S.S.; Elgamal, A.M.; Elshamy, A.I. Oral and Topical Anti-Inflammatory and Antipyretic Potentialities of Araucaria bidiwillii Shoot Essential Oil and Its Nanoemulsion in Relation to Chemical Composition. Molecules 2021, 26, 5833. https://doi.org/10.3390/molecules26195833
Abdelhameed MF, Asaad GF, Ragab TIM, Ahmed RF, El Gendy AE-NG, Abd El-Rahman SS, Elgamal AM, Elshamy AI. Oral and Topical Anti-Inflammatory and Antipyretic Potentialities of Araucaria bidiwillii Shoot Essential Oil and Its Nanoemulsion in Relation to Chemical Composition. Molecules. 2021; 26(19):5833. https://doi.org/10.3390/molecules26195833
Chicago/Turabian StyleAbdelhameed, Mohamed F., Gihan F. Asaad, Tamer I. M. Ragab, Rania F. Ahmed, Abd El-Nasser G. El Gendy, Sahar S. Abd El-Rahman, Abdelbaset M. Elgamal, and Abdelsamed I. Elshamy. 2021. "Oral and Topical Anti-Inflammatory and Antipyretic Potentialities of Araucaria bidiwillii Shoot Essential Oil and Its Nanoemulsion in Relation to Chemical Composition" Molecules 26, no. 19: 5833. https://doi.org/10.3390/molecules26195833