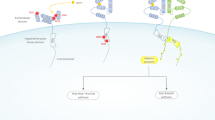
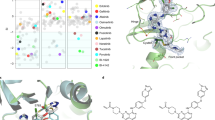
Abstract
Protein tyrosine kinases of the human epidermal growth factor receptor family, including EGFR and HER2, have emerged as important therapeutic targets in non-small-cell lung, breast and gastroesophageal cancers, and are of relevance for the treatment of various other malignancies (particularly colorectal cancer). Classic activating EGFR exon 19 deletions and exon 21 mutations, and HER2 amplification and/or overexpression, are predictive of response to matched molecularly targeted therapies, translating into favourable objective response rates and survival outcomes. By comparison, cancers with insertion mutations in exon 20 of either EGFR or HER2 are considerably less sensitive to the currently available tyrosine kinase inhibitors and antibodies targeting these receptors. These exon 20 insertions are structurally distinct from other EGFR and HER2 mutations, providing an explanation for this lack of sensitivity. In this Review, we first discuss the prevalence and pan-cancer distribution of EGFR and HER2 exon 20 insertions, their biology and detection, and associated responses to current molecularly targeted therapies and immunotherapies. We then focus on novel approaches that are being developed to more effectively target tumours driven by these non-classic EGFR and HER2 alterations.
Key points
-
EGFR and HER2 exon 20 insertion mutations occur in approximately 0.35% and 0.34%, respectively, of all cancers in the American Association for Cancer Research Project GENIE database but are considerably more prevalent in urothelial, non-small-cell lung, breast and central nervous system cancers.
-
Sensitive targeted assays are essential to reliably detect EGFR or HER2 exon 20 insertions.
-
First-generation to third-generation EGFR tyrosine kinase inhibitors (TKIs) and HER2 TKIs have modest activity against cancers with exon 20 insertions in EGFR and HER2, respectively.
-
In 2021, both the EGFR–MET-targeted bispecific antibody amivantamab and the novel EGFR TKI mobocertinib received Accelerated Approval from the FDA for the treatment of patients with advanced-stage non-small-cell lung cancer harbouring EGFR exon 20 insertions.
-
New drugs and combination approaches for patients harbouring EGFR or HER2 exon 20 insertions are under investigation.
-
Little is known about the clinical implications of co-mutations, such as TP53 or STK11 mutations, in cancers with EGFR or HER2 exon 20 insertions, warranting further studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
05 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41571-021-00571-4
References
Malone, E. R., Oliva, M., Sabatini, P. J. B., Stockley, T. L. & Siu, L. L. Molecular profiling for precision cancer therapies. Genome Med. 12, 8 (2020).
Blumenthal, G. M. et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non–small-cell lung cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses. J. Clin. Oncol. 33, 1008–1014 (2015).
Wee, P. & Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 9, 52 (2017).
Oh, D.-Y. & Bang, Y.-J. HER2-targeted therapies – a role beyond breast cancer. Nat. Rev. Clin. Oncol. 17, 33–48 (2020).
Wu, R. et al. A narrative review of advances in treatment and survival prognosis of HER2-positive malignant lung cancers. J. Thorac. Dis. 13, 3708–3720 (2021).
Arcila, M. E. et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 18, 4910–4918 (2012).
Katayama, R. et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci. Transl. Med. 4, 120ra17 (2012).
Mishra, R., Patel, H., Alanazi, S., Yuan, L. & Garrett, J. T. HER3 signaling and targeted therapy in cancer. Oncol. Rev. 12, 355 (2018).
Sharma, N. & Graziano, S. Overview of the LUX-Lung clinical trial program of afatinib for non-small cell lung cancer. Cancer Treat. Rev. 69, 143–151 (2018).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13, 239–246 (2012).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Costa, R. L. B. & Czerniecki, B. J. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 6, 10 (2020).
Liu, H., Zhang, B. & Sun, Z. Spectrum of EGFR aberrations and potential clinical implications: insights from integrative pan-cancer analysis. Cancer Commun. 40, 43–59 (2020).
Kobayashi, Y. & Mitsudomi, T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 107, 1179–1186 (2016).
Hanif, F., Muzaffar, K., Perveen, K., Malhi, S. M. & Simjee, S. U. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 18, 3–9 (2017).
Sequist, L. V., Bell, D. W., Lynch, T. J. & Haber, D. A. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J. Clin. Oncol. 25, 587–595 (2007).
Gristina, V. et al. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: a systematic review and critical appraisal. Cancer Treat. Rev. 85, 101994 (2020).
My Cancer Genome. EGFR exon 20 insertion. https://www.mycancergenome.org/content/alteration/egfr-exon-20-insertion/ (2017).
Oxnard, G. R. et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J. Thorac. Oncol. 8, 179–184 (2013).
Li, Z. et al. A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. EBioMedicine 62, 103074 (2020).
Bang, Y.-J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734 (2015).
Yu, H. A. et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 19, 2240–2247 (2013).
Zhao, J. & Xia, Y. Targeting HER2 alterations in non-small-cell lung cancer: a comprehensive review. JCO Precis. Oncol. 4, 411–425 (2020).
Yoshizawa, A. et al. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer 85, 373–378 (2014).
Connell, C. M. & Doherty, G. J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2, e000279 (2017).
My Cancer Genome. ERBB2 exon 20 insertion. https://www.mycancergenome.org/content/alteration/erbb2-exon-20-insertion (2017).
AACR Project GENIE Consortium.AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 7, 818–831 (2017).
Yasuda, H., Kobayashi, S. & Costa, D. B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 13, e23–e31 (2012).
Beau-Faller, M. et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann. Oncol. 25, 126–131 (2014).
Cardona, A. F. et al. EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer 125, 265–272 (2018).
Robichaux, J. P. et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat. Med. 24, 638–646 (2018).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2019).
Madison, R. W. et al. Urothelial cancer harbours EGFR and HER2 amplifications and exon 20 insertions. BJU Int. 125, 739–746 (2020).
Mazieres, J. et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 31, 1997–2003 (2013).
Robichaux, J. P. et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 36, 444–457.e7 (2019).
Zhao, S. et al. Conformational landscapes of HER2 exon 20 insertions explain their sensitivity to kinase inhibitors in lung adenocarcinoma. J. Thorac. Oncol. 15, 962–972 (2020).
Stephens, P. et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 431, 525–526 (2004).
Rayego-Mateos, S. et al. Role of epidermal growth factor receptor (EGFR) and its ligands in kidney inflammation and damage. Mediators Inflamm. 2018, 8739473 (2018).
Yoshida, T., Zhang, G. & Haura, E. B. Targeting epidermal growth factor receptor: central signaling kinase in lung cancer. Biochem. Pharmacol. 80, 613–623 (2010).
Purba, E. R., Saita, E. I. & Maruyama, I. N. Activation of the EGF receptor by ligand binding and oncogenic mutations: the “rotation model”. Cells 6, 13 (2017).
Riess, J. W. et al. Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J. Thorac. Oncol. 13, 1560–1568 (2018).
Yasuda, H. et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci. Transl. Med. 5, 216ra177 (2013).
Vyse, S. & Huang, P. H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal. Transduct. Target. Ther. 4, 5 (2019).
Eck, M. J. & Yun, C. H. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim. Biophys. Acta 1804, 559–566 (2010).
Galdadas, I. et al. Structural basis of the effect of activating mutations on the EGF receptor. eLife 10, e65824 (2021).
Piotrowska, Z., Wang, Y., Sequist, L. V. & Ramalingam, S. S. ECOG-ACRIN 5162: A phase II study of osimertinib 160mg in NSCLC with EGFR exon 20 insertions [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 9513 (2020).
Kim, T. et al. Phase II study of osimertinib in NSCLC patients with EGFR exon 20 insertion mutation: a multicenter trial of the Korean Cancer Study Group (LU17-19) [abstract 1529P]. Ann. Oncol. 30 (Suppl. 5), v628 (2019).
Graus-Porta, D., Beerli, R. R. & Hynes, N. E. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol. Cell. Biol. 15, 1182–1191 (1995).
Shimamura, T. et al. Non-small-cell lung cancer and Ba/F3 transformed cells harboring the ERBB2 G776insV_G/C mutation are sensitive to the dual-specific epidermal growth factor receptor and ERBB2 inhibitor HKI-272. Cancer Res. 66, 6487–6491 (2006).
Wang, S. E. et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 10, 25–38 (2006).
Perera, S. A. et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc. Natl Acad. Sci. USA 106, 474–479 (2009).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Kosaka, T. et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res. 77, 2712–2721 (2017).
Baraibar, I., Mezquita, L., Gil-Bazo, I. & Planchard, D. Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit. Rev. Oncol. Hematol. 148, 102906 (2020).
Addeo, A., Tabbò, F., Robinson, T., Buffoni, L. & Novello, S. Precision medicine in ALK rearranged NSCLC: a rapidly evolving scenario. Crit. Rev. Oncol. Hematol. 122, 150–156 (2018).
Friedlaender, A., Banna, G., Patel, S. & Addeo, A. Diagnosis and treatment of ALK aberrations in metastatic NSCLC. Curr. Treat. Options Oncol. 20, 79 (2019).
Kitamura, A., Hosoda, W., Sasaki, E., Mitsudomi, T. & Yatabe, Y. Immunohistochemical detection of EGFR mutation using mutation-specific antibodies in lung cancer. Clin. Cancer Res. 16, 3349–3355 (2010).
Zhang, X. et al. HER2 exon 20 insertion mutations in lung adenocarcinoma: case series and response to pyrotinib. Front. Oncol. 10, 1162 (2020).
Angulo, B. et al. A comparison of EGFR mutation testing methods in lung carcinoma: direct sequencing, real-time PCR and immunohistochemistry. PLoS ONE 7, e43842 (2012).
Lindeman, N. I. et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J. Thorac. Oncol. 8, 823–859 (2013).
Lindeman, N. I. et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 142, 321–346 (2018).
Khoo, C., Rogers, T. M., Fellowes, A., Bell, A. & Fox, S. Molecular methods for somatic mutation testing in lung adenocarcinoma: EGFR and beyond. Transl. Lung Cancer Res. 4, 126–141 (2015).
Qiagen. therascreen® EGFR RGQ PCR Kit Handbook Version 2 (Qiagen, 2020).
Yang, S. et al. Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations. Transl. Lung Cancer Res. 10, 753–765 (2021).
Rothberg, J. M. et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature 475, 348–352 (2011).
Skoulidis, F. & Heymach, J. V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 19, 495–509 (2019).
Tuononen, K. et al. Comparison of targeted next-generation sequencing (NGS) and real-time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin-fixed, paraffin-embedded tumor material of non-small cell lung carcinoma-superiority of NGS. Genes Chromosomes Cancer 52, 503–511 (2013).
Coleman, N. et al. EGFR exon 20 insertion (A763_Y764insFQEA) mutant NSCLC is not identified by Roche cobas version 2 tissue testing but has durable intracranial and extracranial response to osimertinib. J. Thorac. Oncol. 15, e162–e165 (2020).
Hwang, I., Kim, S., Chun, S., Yoon, S. & Lee, D. H. Next-generation sequencing for effective detection of various EGFR exon 20 insertions (E20ins) in non-small cell lung cancer (NSCLC) [abstract P2.01-51]. J. Thorac. Oncol. 14 (Suppl. 10), 659 (2019).
Ou, S.-H. I. et al. Characterization of 648 non-small cell lung cancer (NSCLC) cases with 28 unique HER2 exon 20 insertions [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 9063 (2019).
Mack, P. C. et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non-small cell lung cancer: analysis of over 8000 cases. Cancer 126, 3219–3228 (2020).
Food and Drug Administration. FDA grants accelerated approval to amivantamab-vmjw for metastatic non-small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-amivantamab-vmjw-metastatic-non-small-cell-lung-cancer (2021).
FDA. FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20 (2021).
Shu, C. A. et al. CHRYSALIS-2: A phase 1/1b study of lazertinib as monotherapy and in combination with amivantamab in patients with EGFR-mutant NSCLC [abstract]. J. Clin. Oncol. 39 (Suppl. 15), TPS9132 (2021).
Pandey, A. & Brufsky, A. M. Metastatic breast cancer patient with activating HER2 exon 20 insertion mutation with response to poziotinib: case report of compassionate drug use. Clin. Breast Cancer 19, e7–e11 (2019).
Yudong, S. et al. EGFR exon 20 insertion mutation in advanced thymic squamous cell carcinoma: response to apatinib and clinical outcomes. Thorac. Cancer 9, 885–891 (2018).
Voon, P. J., Tsui, D. W. Y., Rosenfeld, N. & Chin, T. M. EGFR exon 20 insertion A763-Y764insFQEA and response to erlotinib–letter. Mol. Cancer Ther. 12, 2614–2615 (2013).
Arcila, M. E. et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol. Cancer Ther. 12, 220–229 (2013).
Yang, G. et al. EGFR exon 20 insertion mutations in Chinese advanced non-small cell lung cancer patients: molecular heterogeneity and treatment outcome from nationwide real-world study. Lung Cancer 145, 186–194 (2020).
Kuiper, J. L. et al. Non-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatment. Br. J. Cancer 115, 1504–1512 (2016).
Choudhury, N. J. et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR exon 20 insertions. Clin. Cancer Res. 27, 2920–2927 (2021).
Costa, D. B. et al. Pulse afatinib for ERBB2 exon 20 insertion-mutated lung adenocarcinomas. J. Thorac. Oncol. 11, 918–923 (2016).
De Greve, J. et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 76, 123–127 (2012).
Mazieres, J. et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann. Oncol. 27, 281–286 (2016).
Besse, B. et al. Neratinib (N) with or without temsirolimus (Tem) in patients (Pts) with non-small cell lung cancer (Nsclc) carrying Her2 somatic mutations: an international randomized phase Ii study [abstract LBA39.PR]. Ann. Oncol. 25 (Suppl. 4), v1 (2014).
Gandhi, L. et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J. Clin. Oncol. 32, 68–75 (2014).
Ma, C. X. et al. Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast cancer. Clin. Cancer Res. 23, 5687–5695 (2017).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703 (2017).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e6 (2018).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Smyth, L. M. et al. Efficacy and determinants of response to HER kinase inhibition in HER2-mutant metastatic breast cancer. Cancer Discov. 10, 198–213 (2020).
Sudhan, D. R. et al. Extended adjuvant therapy with neratinib plus fulvestrant blocks ER/HER2 crosstalk and maintains complete responses of ER(+)/HER2(+) breast cancers: implications to the ExteNET trial. Clin. Cancer Res. 25, 771–783 (2019).
Addeo, A. et al. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat. Rev. 96, 102179 (2021).
Lee, C. K. et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer–a meta-analysis. J. Thorac. Oncol. 12, 403–407 (2017).
Creelan, B. C. et al. A phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br. J. Cancer 124, 383–390 (2020).
Chen, K. et al. Immune microenvironment features and efficacy of PD-1/PD-L1 blockade in non-small cell lung cancer patients with EGFR or HER2 exon 20 insertions. Thorac. Cancer 12, 218–226 (2021).
Negrao, M. V. et al. Association of EGFR and HER-2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non-small cell lung cancer [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 9052 (2018).
Lau, S. C. M. et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin. Lung Cancer 22, 253–259 (2021).
Guisier, F. et al. Efficacy and safety of anti-PD-1 immunotherapy in patients with advanced non small cell lung cancer with BRAF, HER2 or MET mutation or RET-translocation. GFPC 01-2018. J. Thorac. Oncol. 15, 628–636 (2020).
Heymach, J. V. Phase II trial of poziotinib for EGFR and HER2 exon 20 mutant NSCLC. Presented at the IASLC 19th World Conference on Lung Cancer. https://investor.sppirx.com/static-files/d6e05c4b-2c55-4b8c-8d7f-15b6c484f1c9 (2018).
Le, X. et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 9514 (2020).
Sacher, A. et al. Safety, tolerability and preliminary efficacy of poziotinib with twice daily strategy in EGFR/HER2 exon 20 mutant non-small cell lung cancer [abstract 36MO]. Ann. Oncol. 32 (Suppl. 1), 15 (2021).
Socinski, M. A. et al. ZENITH20, a multinational, multi-cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations [abstract LBA60]. Ann. Oncol. 31 (Suppl. 4), 1188 (2020).
Spectrum Pharmaceuticals. FDA grants fast track designation to Spectrum Pharmaceuticals’ poziotinib, https://bwnews.pr/3qCOVW5 (11 March 2021).
Prelaj, A. et al. Poziotinib in advanced NSCLC with EGFR or HER2 exon 20 insertion mutation: initial results from a single site expanded access program [abstract 1388P]. Ann. Oncol. 31 (Suppl. 4), 882 (2020).
Prelaj, A. et al. Poziotinib for EGFR and HER2 exon 20 insertion mutation in advanced NSCLC: results from the expanded access program. Eur. J. Cancer 149, 235–248 (2021).
Russo, A. et al. Heterogeneous responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with uncommon EGFR mutations: new insights and future perspectives in this complex clinical scenario. Int. J. Mol. Sci. 20, 1431 (2019).
Riely, G. J. et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase 1/2 trial. Cancer Discov. 11, 1688–1699 (2021).
Horn, L. et al. Indirect comparison of TAK-788 vs real-world data outcomes in refractory non-small cell lung cancer (NSCLC) with EGFR exon 20 insertions [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 9580 (2020).
Takeda. Takeda announces U.S. FDA Breakthrough Therapy Designation for mobocertinib (TAK-788) for the treatment of NSCLC patients with EGFR exon 20 insertion mutations. https://www.takeda.com/newsroom/newsreleases/2020/takeda-announces-u.s.-fda-breakthrough-therapy-designation-for-mobocertinib-tak-788-for-the-treatment-of-nsclc-patients-with-egfr-exon-20-insertion-mutations/ (2020).
Ramalingam, S. S. et al. Mobocertinib (TAK-788) in EGFR exon 20 insertion (ex20ins)+metastatic NSCLC (mNSCLC): additional results from platinum-pretreated patients (pts) and EXCLAIM cohort of phase 1/2 study [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 9014 (2021).
Zhou, C. et al. Mobocertinib in NSCLC with EGFR Exon 20 insertions: results from EXCLAIM and pooled platinum-pretreated patient populations [abstract OA04.03]. J. Thorac. Oncol. 16 (Suppl. 3), 108 (2021).
Han, H. et al. Targeting HER2 exon 20 insertion-mutant lung adenocarcinoma with a novel tyrosine kinase inhibitor mobocertinib. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-21-1526 (2021).
Estrada-Bernal, A. et al. Antitumor activity of tarloxotinib, a hypoxia-activated EGFR TKI, in patient-derived lung cancer cell lines harboring EGFR exon 20 insertions [abstract]. Mol. Cancer Ther. 17 (Suppl. 1), A157 (2018).
Estrada-Bernal, A. et al. Tarloxotinib is a hypoxia-activated pan-HER kinase inhibitor active against a broad range of HER-family oncogenes. Clin. Cancer Res. 27, 1463–1475 (2021).
Liu, S. et al. First analysis of RAIN-701: study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR exon 20 insertion, HER2-activating mutations & other solid tumours with NRG1/ERBB gene fusions [abstract LBA61]. Ann. Oncol. 31 (Suppl. 4), 1189 (2020).
Chen, Q. et al. Effectiveness and safety of pyrotinib, and association of biomarker with progression-free survival in patients with HER2-positive metastatic breast cancer: a real-world, multicentre analysis. Front. Oncol. 10, 811 (2020).
Ma, F. et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 35, 3105–3112 (2017).
Xu, B. et al. Pyrotinib or lapatinib plus capecitabine for HER2+ metastatic breast cancer (PHOEBE): a randomized phase III trial [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 1003 (2020).
Wang, Y. et al. Comparison the anti-tumor effect of pyrotinib, afatinb and T-DM1 in lung cancer organoids and PDX models with HER2 mutation [abstract]. J. Clin. Oncol. 36 (Suppl. 15), e24281 (2018).
Zhou, C. et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J. Clin. Oncol. 38, 2753–2761 (2020).
Tsigelny, I. F. et al. Molecular determinants of drug-specific sensitivity for epidermal growth factor receptor (EGFR) exon 19 and 20 mutants in non-small cell lung cancer. Oncotarget 6, 6029–6039 (2015).
Brand, T. M., Iida, M. & Wheeler, D. L. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol. Ther. 11, 777–792 (2011).
Wheler, J. J. et al. Combining erlotinib and cetuximab is associated with activity in patients with non-small cell lung cancer (including squamous cell carcinomas) and wild-type EGFR or resistant mutations. Mol. Cancer Ther. 12, 2167–2175 (2013).
Fang, W., Huang, Y., Gan, J., Shao, Y. W. & Zhang, L. Durable response of low-dose afatinib plus cetuximab in an adenocarcinoma patient with a novel EGFR exon 20 insertion mutation. J. Thorac. Oncol. 14, e220–e221 (2019).
Hasegawa, H. et al. Efficacy of afatinib or osimertinib plus cetuximab combination therapy for non-small-cell lung cancer with EGFR exon 20 insertion mutations. Lung Cancer 127, 146–152 (2019).
Fang, W., Huang, Y., Gan, J., Hong, S. & Zhang, L. A patient with EGFR Exon 20 insertion-mutant non-small cell lung cancer responded to osimertinib plus cetuximab combination therapy. J. Thorac. Oncol. 14, e201–e202 (2019).
van Veggel, B. et al. Afatinib and cetuximab in four patients with EGFR exon 20 insertion-positive advanced NSCLC. J. Thorac. Oncol. 13, 1222–1226 (2018).
Goldberg, S. B. et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for first-line treatment of EGFR-mutant non-small-cell lung cancer: final results from SWOG S1403. J. Clin. Oncol. 38, 4076–4085 (2020).
van Veggel, B. et al. Interim results of a phase II single arm trial combining afatinib with cetuximab in patients with EGFRex20ins positive NSCLC [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 9112 (2021).
Khelwatty, S. A. et al. HER2 expression is predictive of survival in cetuximab treated patients with RAS wild type metastatic colorectal cancer. Cancers (Basel) 13, 638 (2021).
Yun, J. et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR–MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 10, 1194–1209 (2020).
Moores, S. L. et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 76, 3942–3953 (2016).
Haura, E. B. et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 9009 (2019).
Park, K. et al. Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 38 (Suppl.), 9512 (2020).
Park, K. et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J. Clin. Oncol. https://doi.org/10.1200/JCO.21.00662 (2021).
Food and Drug Administration. FDA approves first targeted therapy for subset of non-small cell lung cancer. https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-subset-non-small-cell-lung-cancer (2021).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Wang, Y. et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann. Oncol. 30, 447–455 (2019).
Peters, S. et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non–small cell lung cancer: efficacy, safety, and biomarkers. Clin. Cancer Res. 25, 64–72 (2019).
Li, B. T. et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J. Clin. Oncol. 36, 2532–2537 (2018).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Modi, S. et al. Updated results from DESTINY-breast01, a phase 2 trial of trastuzumab deruxtecan (T-DXd) in HER2 positive metastatic breast cancer [abstract]. Cancer Res. 81 (Suppl. 4), PD3-06 (2021).
Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J. Clin. Oncol. 38, 1887–1896 (2020).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Tsurutani, J. et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 10, 688–701 (2020).
Iwata, T. N. et al. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol. Cancer Ther. 17, 1494–1503 (2018).
Mazieres, J. et al. Combination of trastuzumab, pertuzumab and docetaxel in patients with advanced non-small cell lung cancer (NSCLC) harboring HER2 mutation: final results from the IFCT-1703 R2D2 trial [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 9015 (2021).
Butler, L. M., Ferraldeschi, R., Armstrong, H. K., Centenera, M. M. & Workman, P. Maximizing the therapeutic potential of HSP90 inhibitors. Mol. Cancer Res. 13, 1445–1451 (2015).
Kamal, A. et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425, 407–410 (2003).
Jorge, S. E. et al. EGFR exon 20 insertion mutations display sensitivity to Hsp90 inhibition in preclinical models and lung adenocarcinomas. Clin. Cancer Res. 24, 6548–6555 (2018).
Piotrowska, Z. et al. Final results of a phase 2 study of the hsp90 inhibitor luminespib (AUY922) in NSCLC patients harboring EGFR Exon 20 insertions [abstract OA 12.02]. J. Thorac. Oncol. 12 (11 Suppl. 2), 1776 (2017).
Gonzalvez, F. et al. Mobocertinib (TAK-788): a targeted inhibitor of EGFR exon 20 insertion mutants in non-small cell lung cancer. Cancer Discov. 11, 1672–1687 (2021).
Li, A. M., Boichard, A., Felip, E. & Kurzrock, R. New therapeutic approaches to overcoming resistant EGFR exon 20 alterations. Crit. Rev. Oncol. Hematol. 151, 102990 (2020).
Uchibori, K. et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat. Commun. 8, 14768 (2017).
Hasako, S. et al. TAS6417, a novel EGFR inhibitor targeting exon 20 insertion mutations. Mol. Cancer Ther. 17, 1648–1658 (2018).
Piotrowska, Z. et al. Safety and activity of CLN-081 (TAS6417) in NSCLC with EGFR exon 20 insertion mutations (Ins20) [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 9077 (2021).
Schram, A. M. et al. Safety and preliminary efficacy from the phase 1 portion of MasterKey-01: A First-in-human dose-escalation study to determine the recommended phase 2 dose (RP2D), pharmacokinetics (PK) and preliminary antitumor activity of BDTX-189, an inhibitor of allosteric ErbB mutations, in patients (pts) with advanced solid malignancies [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 3086 (2021).
Yang, J. C.-H. et al. Preliminary safety and efficacy results from phase 1 studies of DZD9008 in NSCLC patients with EGFR Exon20 insertion mutations [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 9008 (2021).
Jang, J. et al. Discovery of a highly potent and broadly effective epidermal growth factor receptor and HER2 exon 20 insertion mutant inhibitor. Angew. Chem. 130, 11803–11807 (2018).
Nagamoto, Y. et al. Preclinical evaluation of DS-2087b, a novel and selective inhibitor of EGFR/HER2 exon 20 insertions [abstract 11P]. Ann. Oncol. 31 (Suppl. 4), 248 (2020).
Li, J. et al. A multicenter, open-label, phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of JMT-101 in patients (pts) with advanced colorectal cancer (ACC) [abstract]. J. Clin. Oncol. 38 (Suppl. 15), e16025 (2020).
Kim, H. R. et al. Prediction for response duration to epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR mutated never smoker lung adenocarcinoma. Lung Cancer 83, 374–382 (2014).
Roeper, J. et al. TP53 co-mutations in EGFR mutated patients in NSCLC stage IV: a strong predictive factor of ORR, PFS and OS in EGFR mt+NSCLC. Oncotarget 11, 250–264 (2020).
Stamos, J., Sliwkowski, M. X. & Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 277, 46265–46272 (2002).
Wood, E. R. et al. A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 64, 6652–6659 (2004).
Acknowledgements
We thank the patients, their families and the clinical trial teams who help with drug development. Molecular graphics of PDB structures were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from the NIH (grant P41-GM103311). We thank R. Tufano for annotating variants in genomic databases. The work of V.S. is supported by NIH grant R01CA242845 and by his institution via the NIH Cancer Center Support Grant (P30 CA016672). We acknowledge the American Association for Cancer Research and its financial and material support in the development of the AACR Project GENIE registry, as well as members of the consortium for their commitment to data sharing. Interpretations of the GENIE data are the responsibility of the study authors.
Author information
Authors and Affiliations
Contributions
A.F., V.S., A.R., G.L.B., U.M. and A.A. wrote the manuscript. All authors researched data for the article, contributed to discussions of content, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.F. has received personal fees from Amgen, Astellas, Bayer, Boehringer Ingelheim, Bristol Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Pfizer and Roche. V.S. has received grants from and holds advisory board and/or consultant positions for Eli Lilly/Loxo Oncology. V.S. has also received research grants from Abbvie, Agensys, Alfasigma, Altum, Amgen, Bayer, Berghealth, Blueprint Medicines, Boston Biomedical, Boston Pharmaceuticals, Celgene, D3, Dragonfly Therapeutics, Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharma, Incyte, Inhibrx, MedImmune, Multivir, Nanocarrier, the National Comprehensive Cancer Network, the National Cancer Institute Cancer Therapy Evaluation Program (CTEP), Northwest Biotherapeutics, Novartis, Pharmamar, Pfizer, Roche/Genentech, Takeda, The University of Texas MD Anderson Cancer Center, Turning Point Therapeutics and Vegenics; holds advisory board/consultant positions with Daiichi-Sankyo, Eisai, Helsinn, Incyte, MedImmune, Novartis, QED Pharma, Relay Therapeutics and Signant Health; has received travel funds from ASCO, ESMO, Incyte and Pharmamar; and has received educational seminar support from Medscape. A.R. has received personal fees for attending advisory board meetings from AstraZeneca, MSD and Novartis. G.L.B. has received personal fees from Boehringer Ingelheim, Janssen-Cilag and Roche. U.M. has received personal fees for participation in speakers bureaus and advisory roles from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Merck, MSD, Roche and Thermofisher. C.R. has received funding from the Lung Cancer Research Foundation-Pfizer Grant 2019. C.R. has also received personal fees for attending advisory board meetings from ArcherDx, BMS, Boston Pharmaceuticals, Inivata, MD Serono and Novartis; fees for speakers bureau from AstraZeneca, MSD and Roche; and non-financial support from GuardantHealth through a research collaboration. A.A. has received personal fees for attending advisory board meetings from Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD, Pfizer and Roche; and for speakers bureaus from AstraZeneca, Eli Lilly and MSD.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks K. Park, who co-reviewed with S. Park; F. Cappuzzo; and S. Vyse for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
PDB 1M14: https://www.rcsb.org/structure/1M14
PDB 1XKK: https://www.rcsb.org/structure/1XKK
Supplementary information
Rights and permissions
About this article
Cite this article
Friedlaender, A., Subbiah, V., Russo, A. et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol 19, 51–69 (2022). https://doi.org/10.1038/s41571-021-00558-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00558-1
This article is cited by
-
Oncogenic alterations in advanced NSCLC: a molecular super-highway
Biomarker Research (2024)
-
Progression from ductal carcinoma in situ to invasive breast cancer: molecular features and clinical significance
Signal Transduction and Targeted Therapy (2024)
-
A high affinity and specificity anti-HER2 single-domain antibody (VHH) that targets trastuzumab’s epitope with versatile biochemical, biological, and medical applications
Immunologic Research (2024)
-
EGFR and ERBB2 Exon 20 Insertion Mutations in Chinese Non-small Cell Lung Cancer Patients: Pathological and Molecular Characterization, and First-Line Systemic Treatment Evaluation
Targeted Oncology (2024)
-
Rare oncogenic alterations in NSCLC—focus on atypical EGFR mutations
memo - Magazine of European Medical Oncology (2024)