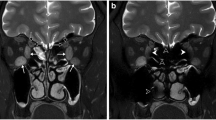
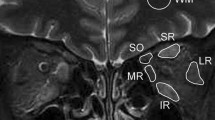
Abstract
Purpose
We aimed to investigate the performance of T1 mapping and its histological correlation with extraocular muscle fibrosis in thyroid-associated ophthalmopathy (TAO).
Methods
We prospectively recruited 12 cases of active TAO, 12 cases of inactive TAO, and 15 cases of control subjects. All participants underwent magnetic resonance imaging (MRI) scan with pre-/postcontrast T1 mapping and short-time inversion-recovery (STIR) sequence. The images were analyzed to obtain precontrast T1, extracellular-volume (ECV) fraction on T1 mapping, and signal-intensity ratio (SIR) on STIR for each rectus. Muscle biopsy was performed at lateral rectus to quantify-collagen volume fraction, glycosaminoglycan (GAG)-volume fraction, and extracellular space component. The relationship between MRI and histopathology was examined with Pearson correlation coefficient.
Results
The active TAO group was characterized with GAG accumulation, while the inactive TAO group presented with substantial fibrosis. The MRI parameters achieved acceptable interobserver and intraobserver agreement. The precontrast T1 and ECV remarkably increased in the TAO groups than the control group, and ECV positively correlated with collagen-volume fraction (r = 0.913) and extracellular-space component (r = 0.886) in the inactive TAO group. The SIR statistically increased in the active TAO group, and SIR positively correlated with GAG-volume fraction in all three groups. The performance of ECV (cutoff > 48.1%) to screen out extraocular muscle fibrosis in inactive TAO was 60.9% sensitivity and 93.3% specificity.
Conclusions
The ECV parameter on T1 mapping MRI is a reliable tool to quantify extraocular muscle fibrosis, providing insights into noninvasive evaluation of pathological changes in TAO orbit.
Trial registration number
ChiCTR2000040394; Date of registration: 28 November 2020



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.
References
R.S. Bahn, Graves’ ophthalmopathy. N. Engl. J. Med. 362(8), 726–738 (2010)
N.M. Hodgson, F. Rajaii, Current understanding of the progression and management of thyroid associated orbitopathy: A systematic review. Ophthalmol. Ther. 9(1), 21–33 (2020)
P.N. Taylor, L. Zhang, R.W.J. Lee, I. Muller, D.G. Ezra, C.M. Dayan, G.J. Kahaly, M. Ludgate, New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat. Rev. Endocrinol. 16(2), 104–116 (2020)
J. Guo, X. Li, R. Ma, J. Qian, Correlation between uniocular deviation and duction changes following different decompression surgeries in thyroid eye disease. BMC Ophthalmol. 21(1), 134 (2021)
R. Ma, H. Ren, B. Xu, Y. Cheng, L. Gan, R. Zhang, J. Wu, J. Qian, PH20 inhibits TGFβ1-induced differentiation of perimysial orbital fibroblasts via hyaluronan-CD44 pathway in thyroid-associated ophthalmopathy. Invest. Ophthalmol. Vis. Sci. 60(5), 1431–1441 (2019)
J. Diao, X. Chen, L. Jiang, P. Mou, R. Wei, Transforming growth factor-β1 suppress pentraxin-3 in human orbital fibroblasts. Endocrine 70(1), 78–84 (2020)
J. Barrio-Barrio, A.L. Sabater, E. Bonet-Farriol, Á. Velázquez-Villoria, J.C. Galofré, Graves’ ophthalmopathy: VISA versus EUGOGO classification. Assess., Manag. J. Ophthalmol. 2015, 249125 (2015)
W. Honglertnapakul, K.M. Cavuoto, C.A. McKeown, H. Capó, Surgical treatment of strabismus in thyroid eye disease: characteristics, dose-response, and outcomes. J. AAPOS 24(2), 72.e1–72.e7 (2020)
M. Baues, A. Dasgupta, J. Ehling, J. Prakash, P. Boor, F. Tacke, F. Kiessling, T. Lammers, Fibrosis imaging: Current concepts and future directions. Adv. Drug Deliv. Rev. 121, 9–26 (2017)
H. Hu, X.Q. Xu, L. Chen, W. Chen, Q. Wu, H.H. Chen, H. Zhu, H.B. Shi, F.Y. Wu, Predicting the response to glucocorticoid therapy in thyroid-associated ophthalmopathy: mobilizing structural MRI-based quantitative measurements of orbital tissues. Endocrine 70(2), 372–379 (2020)
C. Feeney, R.K. Lingam, V. Lee, F. Rahman, S. Nagendran, Non-EPI-DWI for detection, disease monitoring, and clinical decision-making in thyroid eye disease. AJNR Am. J. Neuroradiol. 41(8), 1466–1472 (2020)
F. Tortora, M. Prudente, M. Cirillo, A. Elefante, M.P. Belfiore, F. Romano, S. Cappabianca, C. Carella, S. Cirillo, Diagnostic accuracy of short-time inversion recovery sequence in Graves’ ophthalmopathy before and after prednisone treatment. Neuroradiology 56(5), 353–361 (2014)
R.K. Lingam, P. Mundada, V. Lee, Novel use of non-echo-planar diffusion weighted MRI in monitoring disease activity and treatment response in active Grave’s orbitopathy: An initial observational cohort study. Orbit 37(5), 325–330 (2018)
K. Matsuzawa, S. Izawa, A. Kato, K. Fukaya, K. Matsumoto, T. Okura, D. Miyazaki, M. Kurosaki, S. Fujii, S.I. Taniguchi, M. Kato, K. Yamamoto, Low signal intensities of MRI T1 mapping predict refractory diplopia in Graves’ ophthalmopathy. Clin. Endocrinol. 92(6), 536–544 (2020)
E. Aherne, K. Chow, J. Carr, Cardiac T1 mapping: Techniques and applications. J. Magn. Reson. Imaging 51(5), 1336–1356 (2020)
R.J. Everett, C.G. Stirrat, S.I. Semple, D.E. Newby, M.R. Dweck, S. Mirsadraee, Assessment of myocardial fibrosis with T1 mapping MRI. Clin. Radiol. 71(8), 768–778 (2016)
S. Nakamori, K. Dohi, M. Ishida, Y. Goto, K. Imanaka-Yoshida, T. Omori, I. Goto, N. Kumagai, N. Fujimoto, Y. Ichikawa, K. Kitagawa, N. Yamada, H. Sakuma, M. Ito, Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc. Imaging 11(1), 48–59 (2018)
J. Li, H. Liu, C. Zhang, S. Yang, Y. Wang, W. Chen, X. Li, D. Wang, Native T1 mapping compared to ultrasound elastography for staging and monitoring liver fibrosis: An animal study of repeatability, reproducibility, and accuracy. Eur. Radiol. 30(1), 337–345 (2020)
R. Ma, Y. Cheng, L. Gan, X. Zhou, J. Qian, Histopathologic study of extraocular muscles in thyroid-associated ophthalmopathy coexisting with ocular myasthenia gravis: A case report. BMC Ophthalmol. 20(1), 166 (2020)
V.O. Puntmann, G. Carr-White, A. Jabbour, C.Y. Yu, R. Gebker, S. Kelle, R. Hinojar, A. Doltra, N. Varma, N. Child, T. Rogers, G. Suna, E. Arroyo Ucar, B. Goodman, S. Khan, D. Dabir, E. Herrmann, A.M. Zeiher, E. Nagel, International T1 multicentre CMR outcome study. T1-mapping and outcome in nonischemic cardiomyopathy: All-cause mortality and heart failure. JACC Cardiovasc. Imaging 9(1), 40–50 (2016)
D.H. Hoffman, A. Ayoola, D. Nickel, F. Han, H. Chandarana, K.P. Shanbhogue, T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom. Radio. 45(3), 692–700 (2020)
M. Cheng, M.A. Gromski, E.L. Fogel, J.M. DeWitt, A.A. Patel, T. Tirkes, T1 mapping for the diagnosis of early chronic pancreatitis: Correlation with Cambridge classification system. Br. J. Radiol. 94(1121), 20200685 (2021)
L. Chen, W. Chen, H.H. Chen, Q. Wu, X.Q. Xu, H. Hu, F.Y. Wu, Radiological staging of thyroid-associated ophthalmopathy: Comparison of T1 mapping with conventional MRI. Int. J. Endocrinol. 2020, 2575710 (2020)
S.K. Piechnik, M. Jerosch-Herold, Myocardial T1 mapping and extracellular volume quantification: an overview of technical and biological confounders. Int. J. Cardiovasc. Imaging 34(1), 3–14 (2018)
B.J. Winn, R.C. Kersten, Teprotumumab: Interpreting the clinical trials in the context of thyroid eye disease pathogenesis and current therapies. Ophthalmology S0161-6420(21), 00318–3 (2021)
P. Garg, L.C. Saunders, A.J. Swift, J.M. Wild, S. Plein, Role of cardiac T1 mapping and extracellular volume in the assessment of myocardial infarction. Anatol. J. Cardiol. 19(6), 404–411 (2018)
J.A. Luetkens, S. Klein, F. Traeber, F.C. Schmeel, A.M. Sprinkart, D.L.R. Kuetting, W. Block, K. Hittatiya, F.E. Uschner, R. Schierwagen, J. Gieseke, H.H. Schild, J. Trebicka, G.M. Kukuk, Quantitative liver MRI including extracellular volume fraction for non-invasive quantification of liver fibrosis: A prospective proof-of-concept study. Gut 67(3), 593–594 (2018)
A.J. Taylor, M. Salerno, R. Dharmakumar, M. Jerosch-Herold, T1 mapping: Basic techniques and clinical applications. JACC Cardiovasc. Imaging 9(1), 67–81 (2016)
L.K. McLoon, A. Vicente, K.R. Fitzpatrick, M. Lindström, F. Pedrosa, Domellöf, composition, architecture, and functional implications of the connective tissue network of the extraocular muscles. Invest. Ophthalmol. Vis. Sci. 59(1), 322–329 (2018)
S. Lauer, R.Z. Silkiss, “Stripe sign”- MRI characteristic of extraocular muscles in tendon sparing thyroid eye disease. Orbit (2020) https://doi.org/10.1080/01676830.2020.1782440
M. Zhou, L. Shen, Q. Jiao, L. Ye, Y. Zhou, W. Zhu, W. Wang, S. Wang, Role of magnetic resonance imaging in the assessment of active thyroid-associated ophthalmopathy patients with long disease duration. Endocr. Pract. 25(12), 1268–1278 (2019)
I. Campi, N. Currò, G. Vannucchi, D. Covelli, S. Simonetta, L. Fugazzola, D. Dazzi, L. Pignataro, C. Guastella, E. Lazzaroni, G. Pirola, M. Salvi, Quantification of global ocular motility impairment in Graves’ orbitopathy by measuring eye muscle ductions. Thyroid 31(2), 280–287 (2021)
Acknowledgements
The authors gratefully thank Dr Rui Zhang (Fudan Eye & ENT Hospital), Dr Yanqing Zhang (Fudan Eye & ENT Hospital), and Dr Yifei Yuan (Fudan Eye & ENT Hospital) for providing control cases.
Authors’ contributions
R.M. and YG conceived and designed the study. R.M., Y.G., L.G., and Z.P. performed the study. Y.G., J.C., and J.G. analyzed the data. J.G. and J.Q. supervised the study. R.M. and Y.G. wrote the paper. All authors revised the final version of the paper. J.Q. is the principal investigator of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82000940, 81970835, 81800867) and the Natural Science Foundation of Shanghai (grant number 20ZR1409500).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Approval was obtained from the ethics committee of Fudan Eye & ENT Hospital. The procedures used in this study adhere to the tenets of the Declaration of Helsinki
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Consent to participate
.Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, R., Geng, Y., Gan, L. et al. Quantitative T1 mapping MRI for the assessment of extraocular muscle fibrosis in thyroid-associated ophthalmopathy. Endocrine 75, 456–464 (2022). https://doi.org/10.1007/s12020-021-02873-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02873-0