Abstract
This paper presents a new method for modeling the mechanics of the aortic valve and simulates its interaction with blood. As much as possible, the model construction is based on first principles, but such that the model is consistent with experimental observations. We require that tension in the leaflets must support a pressure, then derive a system of partial differential equations governing its mechanical equilibrium. The solution to these differential equations is referred to as the predicted loaded configuration; it includes the loaded leaflet geometry, fiber orientations and tensions needed to support the prescribed load. From this configuration, we derive a reference configuration and constitutive law. In fluid-structure interaction simulations with the immersed boundary method, the model seals reliably under physiological pressures and opens freely over multiple cardiac cycles. Further, model closure is robust to extreme hypo- and hypertensive pressures. Then, exploiting the unique features of this model construction, we conduct experiments on reference configurations, constitutive laws and gross morphology. These experiments suggest the following conclusions: (1) The loaded geometry, tensions and tangent moduli primarily determine model function. (2) Alterations to the reference configuration have little effect if the predicted loaded configuration is identical. (3) The leaflets must have sufficiently nonlinear material response to function over a variety of pressures. (4) Valve performance is highly sensitive to free edge length and leaflet height. These conclusions suggest appropriate gross morphology and material properties for the design of prosthetic aortic valves. In future studies, our aortic valve modeling framework can be used with patient-specific models of vascular or cardiac flow.


















Similar content being viewed by others
Change history
08 October 2021
In the published article, the Electronic Supplementary Material “10237_2021_1516_MOESM1_ESM.pdf” was inadvertently included. Hence, it has been removed from the article.
References
Abhilash A, Baker B, Trappmann B, Chen C, Shenoy V (2014) Remodeling of fibrous extracellular matrices by contractile cells: predictions from discrete fiber network simulations. Biophys J 107(8):1829–1840
Aggarwal A, Pouch AM, Lai E, Lesicko J, Yushkevich PA, Gorman JH III, Gorman RC, Sacks MS (2016) In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J biomech 49(12):2481–2490
Astorino M, Gerbeau JF, Pantz O, Traore KF (2009) Fluid-structure interaction and multi-body contact: application to aortic valves. Computer Methods Appl Mech Eng 198(45–46):3603–3612
Bao Y, Kaiser AD, Kaye J, Peskin CS (2017) Gaussian-like immersed boundary kernels with three continuous derivatives and improved translational invariance. eprint arXiv:1505.07529v3
Bavo AM, Rocatello G, Iannaccone F, Degroote J, Vierendeels J, Segers P (2016) Fluid-structure interaction simulation of prosthetic aortic valves: comparison between immersed boundary and arbitrary lagrangian-eulerian techniques for the mesh representation. PloS one 11(4):e0154517
Bertoglio C, Caiazzo A, Bazilevs Y, Braack M, Esmaily M, Gravemeier V, Marsden A, Pironneau O, Vignon-Clementel IE, Wall WA (2017) Benchmark problems for numerical treatment of backflow at open boundaries. Int J Numer Methods Biomed Eng. https://doi.org/10.1002/cnm.2918
Billiar KL, Sacks MS (2000) Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: part ii-a structural constitutive model. J Biomech Eng 122(4):327–335
Billiar KL, Sacks MS (2000) Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp-part i: experimental results. J Biomech Eng 122(1):23–30
Boraita A, Heras ME, Morales F, Marina-Breysse M, Canda A, Rabadan M, Barriopedro MI, Varela A, de la Rosa A, Tunon J (2016) Reference values of aortic root in male and female white elite athletes according to sport. Circu Cardiovascular Imag 9(10):e005292
Carretero OA, Oparil S (2000) Essential hypertension: part i: definition and etiology. Circulation 101(3):329–335
Clark RE (1973) Stress-strain characteristics of fresh and frozen human aortic and mitral leaflets and chordae tendineae: implications for clinical use. J Thorac Cardiovascular Surg 66(2):202–208
Clark RE, Swanson W, Kardos JL, Hagen RW, Beauchamp RA (1978) Durability of prosthetic heart valves. Annal Thorac Surg 26(4):323–335
Doose C, Kütting M, Egron S, Farhadi Ghalati P, Schmitz C, Utzenrath M, Sedaghat A, Fujita B, Schmitz-Rode T, Ensminger S, Steinseifer U (2016) Valve-in-valve outcome: design impact of a pre-existing bioprosthesis on the hydrodynamics of an Edwards Sapien XT valve. Eur J Cardio-Thorac Surg 51(3):562–570. https://doi.org/10.1093/ejcts/ezw317
Driessen NJB, Bouten CVC, Baaijens FPT (2004) A structural constitutive model for collagenous cardiovascular tissues incorporating the angular fiber distribution. J Biomech Eng 127(3):494–503. https://doi.org/10.1115/1.1894373
El Khoury G, Vanoverschelde J, Glineur D, Poncelet A, Verhelst R, Astarci P, Underwood M, Noirhomme P (2004) Repair of aortic valve prolapse: experience with 44 patients. Eur J Cardio-Thorac Surg 26(3):628–633. https://doi.org/10.1016/j.ejcts.2004.05.027
Flamini V, DeAnda A, Griffith BE (2016) Immersed boundary-finite element model of fluid-structure interaction in the aortic root. Theor Comput Fluid Dyn 30(1–2):139–164
Gautieri A, Vesentini S, Redaelli A, Buehler MJ (2011) Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano lett 11(2):757–766
Griffith BE (2012) Immersed boundary model of aortic heart valve dynamics with physiological driving and loading conditions. Int J Numer Methods Biomed Eng 28(3):317–345. https://doi.org/10.1002/cnm.1445
Griffith BE (2017) IBAMR: Immersed boundary adaptive mesh refinement. https://github.com/IBAMR/IBAMR
Griffith BE, Hornung RD, McQueen DM, Peskin CS (2010) Parallel and adaptive simulation of cardiac fluid dynamics Adv Comput Infrastruct Parallel Distrib Adapt Appl p 105
Hammer PE, Sacks MS, Pedro J, Howe RD (2011) Mass-spring model for simulation of heart valve tissue mechanical behavior. Annal biomed Eng 39(6):1668–1679
Hasan A, Kolahdouz EM, Enquobahrie A, Caranasos TG, Vavalle JP, Griffith BE (2017) Image-based immersed boundary model of the aortic root. Med Eng Phys 47:72–84
Hsu MC, Kamensky D, Xu F, Kiendl J, Wang C, Wu MC, Mineroff J, Reali A, Bazilevs Y, Sacks MS (2015) Dynamic and fluid-structure interaction simulations of bioprosthetic heart valves using parametric design with t-splines and fung-type material models. Comput Mech 55(6):1211–1225
International Organization for Standardization: ISO 5840-2:2015 cardiovascular implants – cardiac valve prostheses – part 2: Surgically implanted heart valve substitutes. https://www.iso.org/standard/51314.html (2015)
Kaiser AD (2017) Modeling the mitral valve. Ph.D. thesis, Courant Institute of Mathematical Sciences, New York University
Kaiser AD, McQueen DM, Peskin CS (2019) Modeling the mitral valve Int J Numer Methods Biomed Eng p e3240
Kas’yanov V, Purinya B, Ose V (1985) Structure and mechanical properties of the human aortic valve. Mekhanika Kompozitnykh Materialov (MechCompos Mater) 20(5):637–647
Kheradvar A, Groves EM, Falahatpisheh A, Mofrad MK, Alavi SH, Tranquillo R, Dasi LP, Simmons CA, Grande-Allen KJ, Goergen CJ, Baaijens F, Little SH, Canic S, Griffith B (2015) Emerging trends in heart valve engineering: Part iv computational modeling and experimental studies. Annal Biomed Eng 43(10):2314–2333
Kim HJ, Vignon-Clementel IE, Figueroa CA, LaDisa JF, Jansen KE, Feinstein JA, Taylor CA (2009) On coupling a lumped parameter heart model and a three-dimensional finite element aorta model. Annal Biomed Eng 37(11):2153–2169
Laskey WK, Parker HG, Ferrari VA, Kussmaul WG, Noordergraaf A (1990) Estimation of total systemic arterial compliance in humans. J Appl Physiol 69(1):112–119
Lau K, Diaz V, Scambler P, Burriesci G (2010) Mitral valve dynamics in structural and fluid-structure interaction models. Med Eng Phys 32(9):1057–1064
Lee JH, Rygg AD, Kolahdouz EM, Rossi S, Retta SM, Duraiswamy N, Scotten LN, Craven BA, Griffith BE (2020) Fluid–structure interaction models of bioprosthetic heart valve dynamics in an experimental pulse duplicator Annal Biomed Eng pp 1–16
Lim S, Ferent A, Wang X, Peskin C (2008) Dynamics of a closed rod with twist and bend in fluid. SIAM J Scientific Comput 31(1):273–302. https://doi.org/10.1137/070699780
Mao W, Caballero A, McKay R, Primiano C, Sun W (2017) Fully-coupled fluid-structure interaction simulation of the aortic and mitral valves in a realistic 3d left ventricle model. PLOS ONE 12:e0184729. https://doi.org/10.1371/journal.pone.0184729
Marom G, Haj-Ali R, Raanani E, Schäfers HJ, Rosenfeld M (2012) A fluid-structure interaction model of the aortic valve with coaptation and compliant aortic root. Med Biol Eng Comput 50(2):173–182
The Mathworks, Inc., Natick, Massachusetts : MATLAB version 9.3.0.713579 (R2017b) (2017)
May-Newman K, Lam C, Yin FC (2009) A hyperelastic constitutive law for aortic valve tissue J Biomech Eng 131(8)
Peruzzo P, Susin FM, Colli A, Burriesci G (2019) In vitro assessment of pacing as therapy for aortic regurgitation. Open Heart 6(1):e000976
Peskin CS (2002) The immersed boundary method. Acta Numerica 11:479–517
Peskin CS, McQueen DM (1994) Mechanical equilibrium determines the fractal fiber architecture of aortic heart valve leaflets Am J Physiol Heart Circulatory Physiol 266(1), 319–328 http://ajpheart.physiology.org/content/266/1/H319
Pham T, Sulejmani F, Shin E, Wang D, Sun W (2017) Quantification and comparison of the mechanical properties of four human cardiac valves. Acta biomaterialia 54:345–355
Rausch MK, Kuhl E (2013) On the effect of prestrain and residual stress in thin biological membranes. J Mech Phys Solids 61(9):1955–1969
Sacks MS, Smith DB, Hiester ED (1998) The aortic valve microstructure: effects of transvalvular pressure. J Biomed Mater Res: Off J Soc Biomater Jpn Soc Biomater Australian Soc Biomater 41(1):131–141
Sahasakul Y, Edwards WD, Naessens JM, Tajik AJ (1988) Age-related changes in aortic and mitral valve thickness: implications for two-dimensional echocardiography based on an autopsy study of 200 normal human hearts. Am J Cardiol 62(7):424–430
Sathananthan J, Hensey M, Fraser R, Landes U, Blanke P, Hatoum H, Dasi LP, Sedaghat A, Bapat VN, Leipsic J et al (2019) Implications of hydrodynamic testing to guide sizing of self-expanding transcatheter heart valves for valve-in-valve procedures Catheter Cardiovasc Interventions
Sathananthan J, Sellers S, Barlow A, Fraser R, Stanová V, Cheung A, Ye J, Alenezi A, Murdoch DJ, Hensey M et al (2018) Overexpansion of the sapien 3 transcatheter heart valve an ex vivo bench study. JACC Cardiovasc Interventions 11(17):1696–1705
Sauren A (1981) The mechanical behaviour of the aortic valve. Ph.D. thesis, Department of Mechanical Engineering. 10.6100/IR94978
Sauren A, Kuijpers W, van Steenhoven A, Veldpaus F (1980) Aortic valve histology and its relation with mechanics - preliminary report. J Biomech 13(2):97–104
Sauren A, van Hout M, van Steenhoven A, Veldpaus F, Janssen J (1983) The mechanical properties of porcine aortic valve tissues. J Biomech 16(5):327–337
Schäfers HJ, Schmied W, Marom G, Aicher D (2013) Cusp height in aortic valves. J Thorac Cardiovasc Surg 146(2):269–274
Shadden SC, Astorino M, Gerbeau JF (2010) Computational analysis of an aortic valve jet with lagrangian coherent structures. Chaos Interdiscip J Nonlinear Sci 20(1):017512
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78(1):929–958. https://doi.org/10.1146/annurev.biochem.77.032207.120833
Spühler JH, Jansson J, Jansson N, Hoffman J (2018) 3d fluid-structure interaction simulation of aortic valves using a unified continuum ale fem model. Frontiers Physiol 9:363
Stella JA, Sacks MS (2007) On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng 129(5):757–766. https://doi.org/10.1115/1.2768111
Swanson WM, Clark RE (1974) Dimensions and geometric relationships of the human aortic value as a function of pressure. Circ Res 35(6):871–882
Tefft BJ, Choe JA, Young MD, Hennessy RS, Morse DW, Bouchard JA, Hedberg HJ, Consiglio JF, Dragomir-Daescu D, Simari RD et al (2019) Cardiac valve bioreactor for physiological conditioning and hydrodynamic performance assessment. Cardiovasc Eng Technol 10(1):80–94
ViVitro Labs Inc.: Pulse duplicator system user manual. https://vivitrolabs.com/wp-content/uploads/2014/03/Pulse-Duplicator-Manual.pdf (2014)
Yap CH, Kim HS, Balachandran K, Weiler M, Haj-Ali R, Yoganathan AP (2009) Dynamic deformation characteristics of porcine aortic valve leaflet under normal and hypertensive conditions. Am J Physiol-Heart Circ Physiol 298(2):H395–H405
Yellin EL (1989) Dynamics of left ventricular filling. In: Hori M, Suga H, Baan J, Yellin EL (eds) Cardiac mechanics and function in the normal and diseased heart, chap. C. Springer, Tokyo, pp 25–236
Yoganathan AP, Chandran K, Sotiropoulos F (2005) Flow in prosthetic heart valves: state-of-the-art and future directions. Ann Biomed Eng 33(12):1689–1694
Acknowledgements
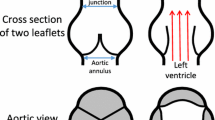
ADK was supported in part by a grant from the National Heart, Lung and Blood Institute (1T32HL098049), Training Program in Mechanisms and Innovation in Vascular Disease at Stanford. ADK and ALM were supported in part by the National Science Foundation SSI Grant #1663671. Computing for this project was performed on the Stanford University’s Sherlock cluster with assistance from the Stanford Research Computing Center. The authors would like to thank Michael Ma for providing the image in Fig. 1, left panel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 38234 kb)
Supplementary file2 (MP4 6492 kb)
Supplementary file3 (MP4 50596 kb)
Appendices
Appendix
A Pressure differences across periodic domains
Here, we show that prescribing a uniform body force on a periodic domain is equivalent to prescribing the value of pressure at the top and bottom of the domain via a change of variables. Let H denote the domain height and P(t) denote the desired pressure difference at time t. Define a body force
Consider a solution of the Navier Stokes momentum equation on a domain periodic in z subject to this body force with modified pressure \(p_{mod}\).
By periodicity, the pressure at the bottom of the domain \(z_{min}\) and top of the domain \(z_{max}\) are equal, or
Now consider the pressure p defined as
The identical velocity field \({\mathbf {u}}\) as in Eq. (36) and the pressure field p then solve the Navier Stokes momentum equation on a nonperiodic domain
and further
Thus, the pressure p has the desired pressure difference across the domain.
B Convergence
Here, we discuss convergence and periodicity of the simulations. We show results at two resolutions. The fine fluid resolution is \(\varDelta x_{fine} = 0.046875\) cm, and the fine structure resolution is targeted to half the fluid resolution, or \(\varDelta s_{fine} = 0.023\) cm. The coarse fluid resolution is twice that of the fine resolution, or \(\varDelta x_{coarse} = 0.09375\) cm, and the coarse structure resolution is targeted to \(\varDelta s_{coarse} = 0.047\) cm. Resolution double the fine resolution is prohibitively expensive to run. Simulations are run for four cardiac cycles, rather than three as in the remainder of the paper.
Precise convergence in IB simulations is challenging to achieve for the following reasons, though we achieve some qualitative and quantitative agreement across cycles and resolutions. Since the IB method uses diffuse interfaces, the structure interacts with fluid points in the support of the discrete delta function, up to \(2.5 \varDelta x\) away. This causes a decrease in the effective orifice area of the valve, even with the same configuration of the structure itself, and thus a slightly lower flow rate. Thus, the fluid/structure domain has a resolution-dependent resistance to forward flow. The aortic pressure is determined by the lumped parameter network, which in turn depends on the flow rate through the three-dimensional model. Precise, simultaneous periodicity of the ODE and three-dimensional flow dynamics is thus challenging to achieve. Additionally, the velocity field has physical instabilities since Reynolds numbers are much larger than one, so precise correspondence of flow fields from cycle to cycle is not expected.
The driving pressure and flow rates are shown in Fig. 19. After the first cardiac cycle, the fine resolution has aortic minimum and maximum pressures of 75–79 and 119–121 mmHg, respectively. The total flow per cycle ranges from 70.7 to 75.5 ml. Due to lack of dramatic changes in flow or pressure from the second through fourth cycles, all other simulations are stopped after three cycles and results are presented during the third cycle. The coarse resolution, due to increased resistance and lower flow rates driving the lumped parameter network, shows a decreasing trend in aortic pressure and a corresponding increase in flow. Thus, at fine resolution the results are closer to periodic in time than at coarse resolution.
The velocity field at fine and coarse resolution during two points in the cardiac cycle is shown in Fig. 20. The valve and flow fields during closure appear similar at both resolutions. The leaflets are closer together in the fine version, and about twice as far apart given the thicker discrete delta function in the coarse version. During forward flow, both flows show a strong jet. Vortices and flow structure appear in the fine resolution jet. The coarse resolution valve opens slightly less, and the effective thickening from the IB method is greater, so the coarse resolution jet is narrowed and has a more uniform appearance. Despite substantially more visible structure in the fine resolution version, both resolutions produce qualitatively similar flow fields.
Rights and permissions
About this article
Cite this article
Kaiser, A.D., Shad, R., Hiesinger, W. et al. A design-based model of the aortic valve for fluid-structure interaction. Biomech Model Mechanobiol 20, 2413–2435 (2021). https://doi.org/10.1007/s10237-021-01516-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01516-7