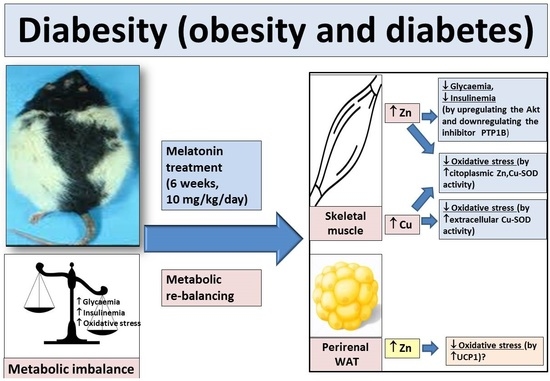
Melatonin Improves Levels of Zn and Cu in the Muscle of Diabetic Obese Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Animals and Experimental Protocols
2.3. Zn and Cu Determination by Flame Atomic Absorption Spectrometry (AAS)
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, G.; Ma, T.; Deng, Z.; Gutiérrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reiter, R.J.; et al. Plant-derived melatonin from food: A gift of nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Otamas, A.; Grant, P.J.; Ajjan, R.A. Diabetes and atherothrombosis: The circadian rhythm and roleof melatonin in vascular protection. Diab. Vasc. Dis. Res. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Int. J. Epidemiol. 2009, 38, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Pietroiusti, A.; Neri, A.; Somma, G.; Coppeta, L.; Iavicoli, I.; Bergamaschi, A.; Magrini, A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup. Environ. Med. 2010, 67, 54–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.R. Sleep and type 2 diabetes mellitus: Clinical implications. J. Assoc. Physicians India 2012, 60, 42–47. [Google Scholar]
- Olds, W. Sleep, Circadian Rhythms, and Metabolism: The Rhythm of Life; Apple Academic Press: Toronto, ON, Canada, 2014. [Google Scholar]
- Gerhart-Hines, Z.; Lazar, M.A. Circadian metabolism in the light of evolution. Endocr. Rev. 2015, 36, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Agil, A.; Rosado, I.; Ruiz, R.; Figueroa, A.; Zen, N.; Fernández-Vázquez, G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J. Pineal Res. 2012, 52, 203–210. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.I.; Huh, J.Y.; Sohn, J.H.; Choe, S.S.; Lee, Y.S.; Lim, C.Y.; Jo, A.; Park, S.B.; Han, W.; Kim, J.B. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol. Cell. Biol. 2015, 35, 1686–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc transporters and insulin resistance: Therapeutic implications for type 2 diabetes and metabolic disease. J. Biomed. Sci. 2017, 24, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS ONE 2018, 13, e0192727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, S.A.; Nield, A.; Chew, G.S.; Myers, M.A. The zinc transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PLoS ONE 2013, 8, e79316. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wenjun, J.; Zhao, M.; Wang, M.; Yan, W.; Chen, M.; Ren, S.; Yuan, B.; Wang, B.; Chen, L. Protamine zinc insulin combined with sodium selenite improves glycometabolism in the diabetic KKAy mice. Sci. Rep. 2016, 6, 26563. [Google Scholar] [CrossRef] [Green Version]
- Eaton, J.W.; Qian, M. Interactions of copper with glycated proteins: Possible involvement in the etiology of diabetic neuropathy. Mol. Cell. Biochem. 2002, 234–235, 135–142. [Google Scholar] [CrossRef]
- Kazi, T.G.; Afridi, H.I.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Kandhro, G.A. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol. Trace Elem. Res. 2008, 122, 1–18. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, D.; Xu, J.; Chi, B. Effect of tauroursodeoxycholic acid and 4-phenylbutyric acid on metabolism of copper and zinc in type 1 diabetic mice model. Biol. Trace Elem. Res. 2016, 170, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, R.K.; Koo, J.R.; Roberts, C.K.; Vaziri, N.D. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: Response to insulin and antioxidant therapies. Clin. Exper. Hypert. 2004, 26, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, D.; Ashokkumar, N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 2013, 95, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, L.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Invest. 2019, 49, e13036. [Google Scholar] [CrossRef] [Green Version]
- Agil, A.; Chayah, M.; Visiedo, L.; Navarro-Alarcon, M.; Rodríguez Ferrer, J.M.; Tassi, M.; Reiter, R.J.; Fernández-Vázquez, G. Melatonin improves mitochondrial dynamics and function in the kidney of zücker diabetic fatty rats. J. Clin. Medic. 2020, 9, 2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Verma, R.; Halda, C. Melatonin ameliorates Bisphenol S induced testicular damages by modulating Nrf-2/HO-1 and SIRT-1/FOXO-1 expressions. Environ. Toxicol. 2021, 36, 396–407. [Google Scholar] [CrossRef]
- Othman, M.S.; Fareid, M.A.; Hameed, R.S.A.; Moneim, A.E.A. The protective effects of melatonin on aluminum-induced hepatotoxicity and nephrotoxicity in rats. Oxid. Medic. Cell. Longev. 2020, 7375136. [Google Scholar]
- Agil, A.; Navarro-Alarcón, M.; Ruiz, R.; Abuhamadah, S.; El-Mir, M.Y.; Vázquez, G.F. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Sanchez-Sanchez, J.J.; Kaski, J.C.; Reiter, R.J. Melatonin and circadian biology in human cardiovascular disease. J. Pineal Res. 2010, 49, 14–22. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Reiter, R.J. Melatonin and cardiovascular disease: Myth or reality? Rev. Esp. Cardiol. 2012, 65, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Reiter, R.; Jiménez-Aranda, A.; Ibán-Arias, R.; Navarro-Alarcón, M.; Marchal, J.A.; Adem, A.; Fernández-Vázque, G. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 2013, 54, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Goettsch, C.; Morawietz, H. Oxidative stress and endothelial dysfunction. Hamostaseologie 2007, 27, 5–12. [Google Scholar] [CrossRef]
- Tamarindo, G.H.; Gobbo, M.G.; Taboga, S.R.; Almeida, E.A.; Góes, R.M. Melatonin ameliorates degenerative alterations caused by age in the rat prostate and mitigates high-fat diet damages. Cell Biol. Intern. 2020, 45, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Tavares, B.S.; Tsosura, T.V.S.; Mattera, M.S.L.C.; Santelli, J.O.; Belardi, B.E.; Chiba, F.Y.; Cintra, L.T.A.; Silva, C.C.; Matsushita, D.H. Effects of melatonin on insulin signaling and inflammatory pathways of rats with apical periodontitis. Intern. Endod. J. 2021, 54, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; Mohammad A.-Serrano, M.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; Agil, A. Antioxidant activity of melatonin in diabetes in relation to the regulation and levels of plasma Cu, Zn, Fe, Mn, and Se in Zucker diabetic fatty rats. Nutrition 2014, 29, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Aouichat, S.; Navarro-Alarcon, M.; Ncir, M.; Zourgui, L.; Agil, A. Melatonin improves endoplasmic reticulum stress–mediated ire1α pathway in zücker diabetic fatty rat. Pharmaceuticals 2021, 14, 232. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Villalón, M.; Jiménez, C.; Quesada-Granados, J.; Agil, A. Melatonin increases magnesium concentrations in white adipose tissue and pancreas of diabetic obese rats. J. Funct. Foods 2018, 48, 167–172. [Google Scholar] [CrossRef]
- Staniek, H.; Rhodes, N.R.; Di Bona, K.R.; Deng, G.; Love, S.T.; Pledger, L.A.; Blount, J.; Gomberg, E.; Grappe, F.; Cernosek, C.; et al. Comparison of tissue metal concentrations in Zucker lean, zucker obese, and zucker diabetic fatty rats and the effects of chromium supplementation on tissue metal concentrations. Biol. Trace El. Res. 2013, 151, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Hosseinzadeh-Attar, M.J.; Alipoor, E.; Sehat, M.; Mohajeri-Tehra, M.R. Effects of zinc supplementation on serum adiponectin concentration and glycemic control in patients with type 2 diabetes. J. Trace Elem. Medic. Biol. 2019, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, R.; Santosh, R.G. A study on relationship between fasting plasma glucose, copper and ceruloplasmin levels in type 2 diabetes mellitus. J. Evol. Med. Dent. Sci. 2015, 4, 11674–11676. [Google Scholar]
- Wu, Y.; Lu, H.; Yang, H.; Li, C.; Sang, Q.; Liu, X.; Liu, Y.; Wang, Y.; Sun, Z. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: Essential roles of Akt-GLUT4, GSK3β and mTOR-S6K1. J. Nutr. Biochem. 2016, 34, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Nagata, Y.; Wada, E.; Zammit, P.S.; Shiozuka, M.; Matsuda, R. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exper. Cell Res. 2015, 333, 228–237. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Sun, W.; Miao, X.; Zhou, S.; Zhang, L.; Epstein, P.N.; Mellen, N.; Zheng, Y.; Fu, y.; Wang, Y.; Cai, L. Zinc rescue of Akt2 gene deletion-linked murine cardiac dysfunction and pathological changes is metallothionein-dependent. J. Mol. Cell Cardiol. 2014, 74, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Adulcikas, J.; Sonda, S.; Norouzi, S.; Sohaland, S.S.; Myers, S. Targeting the zinc transporter ZIP7 in the treatment of insulin resistance and type 2 diabetes. Nutrients 2019, 11, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellomo, E.; Massarotti, A.; Hogstrand, C.; Maret, W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics 2014, 6, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Agil, A.; Elmahallawy, E.K.; Rodríguez Ferrer, J.M.; Adem, A.; Bastak, S.M.; Alabbadi, I.; Fino Solano, Y.A.; Navarro-Alarcón, M. Melatonin increases intracellular calcium in liver, muscle, white adipose tissues and pancreas of diabetic obese rats. Food Funct. 2015, 6, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, S.; Adulcikas, J.; Henstridge, D.C.; Sonda, S.; Sohal, S.S.; Myers, S. The zinc transporter Zip7 is downregulated in skeletal muscle of insulin-resistant cells and in mice fed a high-fat diet. Cells 2019, 8, 663. [Google Scholar] [CrossRef] [Green Version]
- Kotler, M.; Rodriguez, C.; Sáinz, R.M.; Antolín, I.; Menéndez-Peláez, A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J. Pineal Res. 1998, 24, 83–89. [Google Scholar] [CrossRef]
- Venkataraman, P.; Selvakumar, K.; Krishnamoorthy, G.; Muthusami, S.; Rameshkumar, R.; Prakash, S.; Arunakaran, J. Effect of melatonin on PCB (Aroclor 1254) induced neuronal damage and changes in Cu/Zn superoxide dismutase and glutathione peroxidase-4 mRNA expression in cerebral cortex, cerebellum and hippocampus of adult rats. Neurosci. Res. 2010, 66, 189–197. [Google Scholar] [CrossRef]
- Vijayasarathy, K.; Shanthi Naidu, K.; Sastry, B.K.S. Melatonin metabolite 6-sulfatoxymelatonin, Cu/Zn superoxide dismutase, oxidized LDL and malondialdehyde in unstable angina. Intern. J. Cardiol. 2010, 144, 315–317. [Google Scholar] [CrossRef]
- Ghattas, M.H.; Abo-Elmatty, D.M. Association of polymorphic markers of the catalase and superoxide dismutase genes with type 2 diabetes mellitus. DNA Cell Biol. 2012, 31, 1598–1603. [Google Scholar] [CrossRef]
- Sudhahar, V.; Urao, N.; Oshikawa, J.; McKinney, R.D.; Llanos, R.M.; Mercer, J.F.B.; Ushio-Fukai, M.; Fuka, T. Copper transporter ATP7A protects against endothelial dysfunction in type 1 diabetic mice by regulating extracellular superoxide dismutase. Diabetes 2013, 62, 3839–3850. [Google Scholar] [CrossRef] [Green Version]
- Sudhahar, V.; Nazir Okur, M.; Bagi, Z.; O’Bryan, J.p.; Hay, N.; Makino, A.; Patel, V.S.; Phillips, S.A.; Stepp, D.; Ushio-Fukai, M.; et al. Akt2 (protein kinase B beta) stabilizes ATP7A, a copper transporter for extracellular superoxide dismutase, in vascular smooth muscle: Novel mechanism to limit endothelial dysfunction in type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esparza, J.L.; Gomez, M.; Rosa Nogues, M.; Paternain, J.L.; Mallol, J.; Domingo, J.L. Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J. Pineal Res. 2005, 39, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular mechanisms of melatonin-mediated cell protection and signaling in health and disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Molpeceres, V.; Garcia-Mediavilla, M.V.; González, P.; Barrio, J.P.; González-Gallego, J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J. Pineal Res. 2007, 42, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, N.Z.; Feizi, A.; Andersen, E.S.; Heywood, S.; Hattel, E.B.; Daugaard, S.; Peijs, L.; Bagi, P.; Feldt-Rasmussen, B.; Schultz, H.S.; et al. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Molec. Metab. 2019, 24, 30–43. [Google Scholar] [CrossRef]
- Jiménez-Aranda, A.; Fernández-Vázquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Balaz, M.; Vician, M.; Janakova, Z.; Kurdiova, T.; Surova, M.; Imrich, R.; Majercikova, Z.; Penesova, A.; Vlcek, M.; Kiss, A.; et al. Subcutaneous adipose tissue zinc-α2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity 2014, 22, 1821–1829. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Alarcón, M.; Gil-Hernández, F.; Sánchez-González, C.; Llopis, J.; Villalón-Mir, M.; Olmedo, P.; Alarcón-Guijo, P.; Salagre, D.; Gaona, L.; Paredes, M.; et al. Melatonin Improves Levels of Zn and Cu in the Muscle of Diabetic Obese Rats. Pharmaceutics 2021, 13, 1535. https://doi.org/10.3390/pharmaceutics13101535
Navarro-Alarcón M, Gil-Hernández F, Sánchez-González C, Llopis J, Villalón-Mir M, Olmedo P, Alarcón-Guijo P, Salagre D, Gaona L, Paredes M, et al. Melatonin Improves Levels of Zn and Cu in the Muscle of Diabetic Obese Rats. Pharmaceutics. 2021; 13(10):1535. https://doi.org/10.3390/pharmaceutics13101535
Chicago/Turabian StyleNavarro-Alarcón, Miguel, Fernando Gil-Hernández, Cristina Sánchez-González, Juan Llopis, Marina Villalón-Mir, Pablo Olmedo, Pablo Alarcón-Guijo, Diego Salagre, Lorena Gaona, Mario Paredes, and et al. 2021. "Melatonin Improves Levels of Zn and Cu in the Muscle of Diabetic Obese Rats" Pharmaceutics 13, no. 10: 1535. https://doi.org/10.3390/pharmaceutics13101535