Abstract
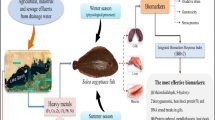
The aim of the present study is the first to evaluate the ecotoxic state of the marine environment in Anza-Taghazout coasts (Morocco) after installation of two wastewater treatment plants using a natural population of marine bivalves Mytilus galloprovincialis. These coasts are exposed to many discharges generating, thus, different sources of pollutants. These pollutants can modulate the physiological responses of marine bivalves to environmental stress. In this context, a multibiomarker approach consisting of a battery of biomarker evaluation was used to assess the response of these species to stress. In the whole soft tissues of M. galloprovincialis, four biomarkers were evaluated as follows: acetylcholinesterase (AChE), glutathione S-transferase (GST), catalase (Cat), and malondialdehyde activity (MDA). In parallel, physico-chemical parameters were measured in the marine water of Anza-Taghazout within three selected sites: S1 considered as “hotspot” located at Anza city; S2 located near of Aourir city; and the third site, S3 “reference” located in Imouran beach. Our results showed that activities of both glutathione S-transferase and catalase were higher in M. galloprovincialis collected from site S1, but high values of malondialdehyde and acetylcholinesterase activities were observed successively at S3 and S2. Application of integrated biomarker response (IBR) index was suitable for classifying the stress response in the M. galloprovincialis but did not allow to evaluate the level of the xenobiotic exposure in the studied sites. The statistical results did not show any significant differences between the three studied sites, and therefore, S1 has recently become clean due to the installation of two wastewater treatment plants.




Similar content being viewed by others
References
Abbassi M, Banaoui A, Kaaya A, Elkhou A, Nadir M, Lefrere L (2015) Biomarker approach to the assessment of the health status of Moroccan marine ecosystems: preliminary study in Sidi Ifni coast (south of Morocco). J Mater Environ Sci 6(11):3086–3093
Aebi HE (1983) Catalase. Methods of enzymatic analysis
Agnaou M, Ait Alla A, Ouassas M, Bazzi L, El Alami Z, Moukrim A (2014) Assessment of organochlorine pesticides contamination of Oued Souss estuary (south of Morocco): seasonal variability in sediment and a detritivore annelid Neries diversicolor. J Mater Environ Sci 5(2):581–586
Agnaou M, Nadir M, Alla AA, Bazzi L, El Alami Z, Moukrim A (2018) The occurrence and spatial distribution of pesticides in sea water of the Agadir bay (south of Morocco). Ecosystems 13:14
Ait Alla A, Mouneyrac C, Durou C, Moukrim A, Pellerin J (2006) Tolerance and biomarkers as useful tools for assessing environmental quality in the Oued Souss estuary (Bay of Agadir, Morocco). Comp Biochem Physiol C 143:23–29. https://doi.org/10.1016/j.cbpc.2005.11.015
Alexandrova ML, Bochev PG (2005) Oxidative stress during the chronic phase after stroke. Free Radic Biol Med 39(3):297–316. https://doi.org/10.1016/j.freeradbiomed.2005.04.017
Almeida EA, Bainy ACD, Dafre AL, Gomes OF, Medeiros MH, Di Mascio P (2005) Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J Exp Mar Biol Ecol 318(1):21–30. https://doi.org/10.1016/j.jembe.2004.12.007
Banaoui A, Chiffoleau JF, Moukrim A, Burgeot T, Kaaya A, Auger D, Rozuel E (2004) Trace metal distribution in the mussel Perna perna along the Moroccan coast. Mar Pollut Bull 48(3-4):385–390. https://doi.org/10.1016/j.marpolbul.2003.11.007
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem Int J 21(6):1316–1322. https://doi.org/10.1016/j.aquatox.2014.01.004
Beyer J, Green NW, Brooks S, Allan IJ, Ruus A, Gomes T, Bråte ILN, Schøyen M (2017) Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: a review. Mar Environ Res 130:338–365. https://doi.org/10.1016/j.marenvres.2017.07.024
Blaise C, Gagné F, Pellerin J, Hansen PD, Trottier S (2002) Molluscan shellfish biomarker study of the Quebec, Canada, Saguenay Fjord with the soft-shell clam, Mya arenaria. Environ Toxicol Int J 17(3):170–186. https://doi.org/10.1002/tox.10048
Blanchette B, Feng X, Singh BR (2007) Marine glutathione S-transferases. Mar Biotechnol 9(5):513–542. https://doi.org/10.1007/s10126-007-9034-0
Bocquené G, Galgani F (1991) L’acétylcholinestérase chez les organismes marins, outil de surveillance des effets des pesticides organophosphorés et carbamates. Oceanis 17:439–448. https://doi.org/10.1051/alr:2004033
Bocquené G C, Galgani F (1998) Biological effects of contaminants: cholinesterase inhibition by organophosphate and carbamate compounds (pp. 1-13). Copenhagen, Denmark: International Council for the Exploration of the Sea
Bocquené G, Chantereau S, Clérendeau C, Beausir E, Ménard D, Raffin B, Minier C, Burgeot T, Leszkowicz AP, Narbonne JF (2004) Biological effects of the “Erika” oil spill on the common mussel (Mytilus edulis). Aquat Living Resour 17(3):309–316. https://doi.org/10.1051/alr:2004033
Bouhallaoui M, Zbiry M, Elkhiati N, Talba S, Sforzini S, Viarengo A, Benhra A (2017) Use of biomarkers to evaluate the effects of environmental stressors on Mytilus galloprovincialis sampled along the Moroccan coasts: integrating biological and chemical data. Mar Environ Res 130:60–68. https://doi.org/10.1016/j.marenvres.2017.05.010
Broeg K, Lehtonen KK (2006) Indices for the assessment of environmental pollution of the Baltic Sea coasts: integrated assessment of a multi-biomarker approach. Mar Pollut Bull 53(8–9):508–522
Canesi L, Borghi C, Ciacci C, Fabbri R, Vergani L, Gallo G (2007) Bisphenol-A alters gene expression and functional parameters in molluscan hepatopancreas. Mol Cell Endocrinol 276(1-2):36–44. https://doi.org/10.1016/j.mce.2007.06.002
Canesi L, Borghi C, Ciacci C, Fabbri R, Lorusso LC, Vergani L, Marcomini A, Poiana G (2008) Short-term effects of environmentally relevant concentrations of EDC mixtures on Mytilus galloprovincialis digestive gland. Aquat Toxicol 87(4):272–279. https://doi.org/10.1016/j.aquatox.2008.02.007
Chaouay A, Bazzi L, Hilali M, Alla AA, El Mouaden K (2014) Study of bacterial contamination of the Bay of Agadir impact on the resistance of copper’s corrosion. J Mater Environ Sci 5:2472–2477
Coppola F, Almeida Â, Henriques B, Soares AM, Figueira E, Pereira E, Freitas R (2017) Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Sci Total Environ 601:1129–1138. https://doi.org/10.1016/j.scitotenv.2017.05.201
Cossu C, Doyotte A, Babut M, Exinger A, Vasseur P (2000) Antioxidant biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicol Environ Saf 45(2):106–121. https://doi.org/10.1006/eesa.1999.1842
Cravo A, Lopes B, Serafim Â, Barreira L, Gomes T, Bebianno MJ (2009) A multibiomarker approach in Mytilus galloprovincialis to assess environmental quality. J Environ Monit 11(9):1673–1686. https://doi.org/10.1039/B909846A
Damiens G, Gnassia-Barelli M, Loquès F, Roméo M, Salbert V (2007) Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere 66(3):574–583. https://doi.org/10.1016/j.chemosphere.2006.05.032
Devin S, Burgeot T, Giambérini L, Minguez L, Pain-Devin S (2014) The integrated biomarker response revisited: optimization to avoid misuse. Environ Sci Pollut Res 21(4):2448–2454. https://doi.org/10.1007/s11356-013-2169-9
Doran WJ, Cope WG, Rada RG, Sandheinrich MB (2001) Acetylcholinesterase inhibition in the three-ridge mussel (Amblema plicata) by chlorpyrifos: implications for biomonitoring. Ecotoxicol Environ Saf 49(1):91–98. https://doi.org/10.1006/eesa.2000.2036
Downs CA, Shigenaka G, Fauth JE, Robinson CE, Huang A (2002) Cellular physiological assessment of bivalves after chronic exposure to spilled Exxon Valdez crude oil using a novel molecular diagnostic biotechnology. Environ Sci Technol 36(13):2987–2993. https://doi.org/10.1021/es011433k
Duarte CA, Giarratano E, Amin OA, Comoglio LI (2011) Heavy metal concentrations and biomarkers of oxidative stress in native mussels (Mytilus edulis chilensis) from Beagle Channel coast (Tierra del Fuego, Argentina). Mar Pollut Bull 62(8):1895–1904. https://doi.org/10.1016/j.marpolbul.2011.05.031
El Mourabit Y, Ait Alla A, Bihadassen B, Moukrim A (2020) 2020.Impact of the installation of two WWTPs on the microbiological quality of sea water in the Anza-Taghazout coasts (southwest of Morocco). Am J Innov Res Appl Sci 11(3):143–153
Elazzaoui A, Moukrim A, Lefrere L (2019) A multibiomarker approach to assess the health state of coastal ecosystem receiving desalination plants in Agadir Bay, Morocco. Sci World J 2019:1–9. https://doi.org/10.1155/2019/5875027
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Fairbrother A, Bennett RS, Bennett JK (1989) Sequential sampling of plasma cholinesterase in mallards (Anas platyrhynchos) as an indicator of exposure to cholinesterase inhibitors. Environ Toxicol Chem Int J 8(2):117–122
Frenzilli G, Nigro M, Scarcelli V, Gorbi S, Regoli F (2001) DNA integrity and total oxyradical scavenging capacity in the Mediterranean mussel, Mytilus galloprovincialis: a field study in a highly eutrophicated coastal lagoon. Aquat Toxicol 53(1):19–32. https://doi.org/10.1016/S0166-445X(00)00159-4
Frouin H, Pellerin J, Fournier M, Pelletier E, Richard P, Pichaud N, Rouleau C, Garnerot F (2007) Physiological effects of polycyclic aromatic hydrocarbons on soft-shell clam Mya arenaria. Aquat Toxicol 82(2):120–134. https://doi.org/10.1016/j.aquatox.2007.02.005
Funes V, Alhama J, Navas JI, López-Barea J, Peinado J (2006) Ecotoxicological effects of metal pollution in two mollusc species from the Spanish South Atlantic littoral. Environ Pollut 139(2):214–223. https://doi.org/10.1016/j.envpol.2005.05.016
Gonçalves-Soares D, Zanette J, Yunes JS, Yepiz-Plascencia GM, Bainy AC (2012) Expression and activity of glutathione S-transferases and catalase in the shrimp Litopenaeus vannamei inoculated with a toxic Microcystis aeruginosa strain. Mar Environ Res 75:54–61. https://doi.org/10.1016/j.marenvres.2011.07.007
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D'Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EM, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952. https://doi.org/10.1126/science.1149345
Hutchinson TH, Lyons BP, Thain JE, Law RJ (2013) Evaluating legacy contaminants and emerging chemicals in marine environments using adverse outcome pathways and biological effects-directed analysis. Mar Pollut Bull 74(2):517–525. https://doi.org/10.1016/j.marpolbul.2013.06.012
Idhalla M, Bouhaimi A, Zekhnini A, Narbonne JF, Mathieu M (1997) Etude du cycle de reproduction de deux espèces de moules Perna perna (Linné, 1758) et Mytilus galloprovincialis Lamarck, 1819 dans la baie d’Agadir (Sud du Maroc). Haliotis (Paris) 26:51–62
Kaaya A, Najimi S, Ribera D, Narbonne JF, Moukrim A (1999) Characterization of glutathione S-transferases (GST) activities in Perna perna and Mytilus galloprovincialis used as a biomarker of pollution in the Agadir marine bay (south of Morocco). Bull Environ Contam Toxicol 62(5):623–629. https://doi.org/10.1007/s001289900920
Kashiwagi A, Kashiwagi K, Takase M, Hanada H, Nakamura M (1997) Comparison of catalase in diploid and haploid Rana rugosa using heat and chemical inactivation techniques. Comp Biochem Physiol B: Biochem Mol Biol 118(3):499–503. https://doi.org/10.1016/S0305-0491(97)00216-2
Kirby MF, Morris S, Hurst M, Kirby SJ, Neall P, Tylor T, Fagg A (2000) The use of cholinesterase activity in flounder (Platichthys flesus) muscle tissue as a biomarker of neurotoxic contamination in UK estuaries. Mar Pollut Bull 40(9):780–791
Labrot F, Ribera D, Denis MS, Narbonne JF (1996) In vitro and in vivo studies of potential biomarkers of lead and uranium contamination: lipid peroxidation, acetylcholinesterase, catalase and glutathione peroxidase activities in three non-mammalian species. Biomarkers 1(1):21–28. https://doi.org/10.3109/13547509609079343
Lagadic L, Caquet T, Amiard JC, Ramade F (eds) (1997) Biomarqueurs en Ecotoxicologie. Aspects Fondamentaux, Masson, Paris, 419 pp
Livingstone DR, Nasci C, Solé M, Da Ros L, O'Hara SC, Peters LD, Fossato V, Wootton AN, Goldfarb PS (1997) Apparent induction of a cytochrome P450 with immunochemical similarities to CYP1A in digestive gland of the common mussel (Mytilus galloprovincialis L.) with exposure to 2, 2′, 3, 4, 4′, 5′-hexachlorobiphenyl and Arochlor 1254. Aquat Toxicol 38(4):205–224. https://doi.org/10.1016/S0166-445X(96)00847-8
Lowe DM, Fossato VU (2000) The influence of environmental contaminants on lysosomal activity in the digestive cells of mussels (Mytilus galloprovincialis) from the Venice Lagoon. Aquat Toxicol 48(2-3):75–85. https://doi.org/10.1016/S0166-445X(99)00054-5
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mimouni R, Ait Alla A, Anajjar EA, Finance C, Moukrim A (2002) Impact of wastewater discharges on the microbiological quality of beaches in the Agadir Bay (Morocco). Journal Européen d'Hydrologie (France)
Moreira A, Figueira E, Soares AM, Freitas R (2016) Salinity influences the biochemical response of Crassostrea angulata to arsenic. Environ Pollut 214:756–766
Moreira A, Freitas R, Figueira E, Ghirardini AV, Soares AM, Radaelli M et al (2018) Combined effects of arsenic, salinity and temperature on Crassostrea gigas embryotoxicity. Ecotoxicol Environ Saf 147:251–259
Moukrim A, Kaaya A, Najimi S, Roméo M, Gnassia-Barelli M, Narbonne JF (2000) Assessment of the trace metal levels in two species of mussels from the Agadir Marine Bay, south of Morocco. Bull Environ Contam Toxicol 65(4):478–485. https://doi.org/10.1007/s001280000149
Orbea A, Cajaraville MP (2006) Peroxisome proliferation and antioxidant enzymes in transplanted mussels of four Basque estuaries with different levels of polycyclic aromatic hydrocarbon and polychlorinated biphenyl pollution. Environ Toxicol Chem Int J 25(6):1616–1626. https://doi.org/10.1897/04-520R2.1
Oruc EO, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2, 4-D and azinphosmethyl. Comp Biochem Physiol C Toxicol Pharmacol 137(1):43–51. https://doi.org/10.1016/j.cca.2003.11.006
Perić L, Burić P (2019) The effect of copper and chlorpyrifos co-exposure on biomarkers in the marine mussel Mytilus galloprovincialis. Chemosphere 225:126–134. https://doi.org/10.1016/j.chemosphere.2019.03.003
Pfeifer S, Schiedek D, Dippner JW (2005) Effect of temperature and salinity on acetylcholinesterase activity, a common pollution biomarker, in Mytilus sp. from the south-western Baltic Sea. J Exp Mar Biol Ecol 320(1):93–103. https://doi.org/10.1016/j.jembe.2004.12.020
Porte C, Sole M, Albaiges J, Livingstone DR (1991) Responses of mixed-function oxygenase and antioxidase enzyme system of Mytilus sp. to organic pollution. Comp Biochem Physiol C Comp Pharmacol Toxicol 100(1-2):183. https://doi.org/10.1016/0742-8413(91)90150-r
Quintaneiro C, Ranville J, Nogueira AJA (2015) Effects of the essential metals copper and zinc in two freshwater detritivores species: biochemical approach. Ecotoxicol Environ Saf 118:37–46. https://doi.org/10.1016/j.ecoenv.2015.04.006
Rank J, Lehtonen KK, Strand J, Laursen M (2007) DNA damage, acetylcholinesterase activity and lysosomal stability in native and transplanted mussels (Mytilus edulis) in areas close to coastal chemical dumping sites in Denmark. Aquat Toxicol 84(1):50–61. https://doi.org/10.1016/j.aquatox.2007.05.013
Regoli F, Principato G (1995) Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat Toxicol 31(2):143–164. https://doi.org/10.1016/0166-445X(94)00064-W
Regoli F, Gorbi S, Frenzilli G, Nigro M, Corsi I, Focardi S, Winston GW (2002) Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Mar Environ Res 54(3-5):419–423. https://doi.org/10.1016/S0141-1136(02)00146-0
Reichwaldt ES, Ghadouani A (2016) Can mussels be used as sentinel organisms for characterization of pollution in urban water systems? Hydrol Earth Syst Sci 20(7):2679–2689. https://doi.org/10.5194/hess-20-2679-2016
Rickwood CJ, Galloway TS (2004) Acetylcholinesterase inhibition as a biomarker of adverse effect: a study of Mytilus edulis exposed to the priority pollutant chlorfenvinphos. Aquat Toxicol 67(1):45–56. https://doi.org/10.1016/j.aquatox.2003.11.004
Rodríguez-Fuentes G, Gold-bouchot G (2004) Characterization of cholinesterase activity from different tissues of Nile tilapia (Oreochromis niloticus). Mar Environ Res 58(2-5):505–509. https://doi.org/10.1016/j.marenvres.2004.03.037
Sáenz LA, Seibert EL, Zanette J, Fiedler HD, Curtius AJ, Ferreira JF, de Almeida EA, Marques MRF, Bainy ACD (2010) Biochemical biomarkers and metals in Perna perna mussels from mariculture zones of Santa Catarina, Brazil. Ecotoxicol Environ Saf 73(5):796–804. https://doi.org/10.1016/j.ecoenv.2010.02.015
Sanchez W, Burgeot T, Porcher JM (2013) A novel “integrated biomarker response” calculation based on reference deviation concept. Environ Sci Pollut Res 20(5):2721–2725. https://doi.org/10.1007/s11356-012-1359-1
Schettino T, Caricato R, Calisi A, Giordano ME, Lionetto MG (2012) Biomarker approach in marine monitoring and assessment: new insights and perspectives. Open Environ Sci 6(1):20–27. https://doi.org/10.2174/1876325101206010020
SGIMC I (2011) Report of the study group on integrated monitoring of contaminants and biological effects (SGIMC) 14–18 March, Copenhagen. Denmark, ICES CM
Sheehan D, Power A (1999) Effects of seasonality on xenobiotic and antioxidant defence mechanisms of bivalve molluscs. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 123(3):193–199. https://doi.org/10.1016/S0742-8413(99)00033-X
Sheehan D, MEADE G, FOLEY VM, DOWD CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360(1):1–16. https://doi.org/10.1042/bj3600001
Simpson CD, Mosi AA, Cullen WR, Reimer KJ (1996) Composition and distribution of polycyclic aromatic hydrocarbon contamination in surficial marine sediments from Kitimat Harbor, Canada. Sci Total Environ 181(3):265–278. https://doi.org/10.1016/0048-9697(95)05026-4
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer 19(3):201–213
Sunderman FW, Marzouk A, Hopfer SM, Zaharia O, Reid MC (1985) Increased lipid peroxidation in tissues of nickel chloride-treated rats. Ann Clin Lab Sci 15(3):229–236
Taleb ZM, Benghali S, Kaddour A, Boutiba Z (2007) Monitoring the biological effects of pollution on the Algerian west coast using mussels Mytilus galloprovincialis. Oceanologia 49(4)
Taleb ZM, Benali I, Gherras H, Ykhlef-Allal A, Bachir-Bouiadjra B, Amiard JC, Boutiba Z (2009) Biomonitoring of environmental pollution on the Algerian west coast using caged mussels Mytilus galloprovincialis. Oceanologia 51(1):63–84
Trisciani A, Perra G, Caruso T, Focardi S, Corsi I (2012) Phase I and II biotransformation enzymes and polycyclic aromatic hydrocarbons in the Mediterranean mussel (Mytilus galloprovincialis, Lamarck, 1819) collected in front of an oil refinery. Mar Environ Res 79:29–36. https://doi.org/10.1016/j.marenvres.2012.04.006
Tsangaris C, Kormas K, Strogyloudi E, Hatzianestis I, Neofitou C, Andral B, Galgani F (2010) Multiple biomarkers of pollution effects in caged mussels on the Greek coastline. Comp Biochem Physiol C Toxicol Pharmacol 151(3):369–378. https://doi.org/10.1016/j.cbpc.2009.12.009
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Viarengo A, Pertica M, Canesi L, Accomando R, Mancinelli G, Orunesu M (1989) Lipid peroxidation and level of antioxidant compounds (GSH, vitamin E) in the digestive glands of mussels of three different age groups exposed to anaerobic and aerobic conditions. Mar Environ Res 28(1-4):291–295. https://doi.org/10.1016/0141-1136(89)90246-8
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant induced stress syndrome in sentinel organisms. Comp Biochem Physiol Part C 146:281–300. https://doi.org/10.1016/j.cbpc.2007.04.011
Vidal ML, Bassères A, Narbonne JF (2002) Influence of temperature, pH, oxygenation, water-type and substrate on biomarker responses in the freshwater clam Corbicula fluminea (Müller). Comp Biochem Physiol C Toxicol Pharmacol 132(1):93–104
Vidal-Liñán L, Bellas J, Campillo JA, Beiras R (2010) Integrated use of antioxidant enzymes in mussels, Mytilus galloprovincialis, for monitoring pollution in highly productive coastal areas of Galicia (NW Spain). Chemosphere 78:265–272. https://doi.org/10.1016/j.chemosphere.2009.10.060
Vidal-Liñán L, Bellas J, Etxebarria N, Nieto O, Beiras R (2014) Glutathione S-transferase, glutathione peroxidase and acetylcholinesterase activities in mussels transplanted to harbour areas. Sci Total Environ 470:107–116. https://doi.org/10.1016/j.scitotenv.2013.09.073
Wepener V, Bervoets L, Mubiana V, Blust R (2008) Metal exposure and biological responses in resident and transplanted blue mussels (Mytilus edulis) from the Scheldt estuary. Mar Pollut Bull 57(6-12):624–631. https://doi.org/10.1016/j.marpolbul.2008.03.030
Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE (2000) Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol 30(4):347–570. https://doi.org/10.1080/10408440091159239
Wilhelm Filho D, Tribess T, Gaspari C, Claudio FD, Torres MA, Magalhaes ARM (2001) Seasonal changes in antioxidant defences of the digestive gland of the brown mussel (Perna perna). Aquaculture 203(1-2):149–158. https://doi.org/10.1016/S0044-8486(01)00599-3
Winston GW, Moore MN, Kirchin MA, Soverchia C (1996) Production of reactive oxygen species by hemocytes from the marine mussel, Mytilus edulis: lysosomal localization and effect of xenobiotics. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 113(2):221–229. https://doi.org/10.1016/0742-8413(95)02091-8
Acknowledgements
Our gratitude is expressed to Prof. Saïd EL Madidi for his statistical advices and Prof. Youssef El Ouidani for grammatical language correction.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, Youssef El Mourabit, upon reasonable request.
Author information
Authors and Affiliations
Contributions
All the authors declare to have participated in this study, with the following participation: Youssef El Mourabit, Abdellatif Moukrim, and Aicha Ait Alla: experimental design and methodology, Youssef El Mourabit and Mustapha Agnaou: experiment assays and analysis, all authors: writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“Not applicable” in this section.
Consent for publication
I give my consent for the publication of details of this work in the above journal and article.
Competing interests
All the authors of the present article declare to have no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations. The following article has not been published previously and that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the copyright holder.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
EL Mourabit, Y., Agnaou, M., Ait Alla, A. et al. Assessment of the marine ecotoxic state in the Moroccan coastal area Anza-Taghazout following the installation of two wastewater treatment plants: a multibiomarker study using Mytilus galloprovincialis. Environ Sci Pollut Res 29, 11718–11729 (2022). https://doi.org/10.1007/s11356-021-16046-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16046-z