Abstract
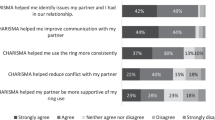
Biomedical, female-initiated HIV prevention methods can help reduce disproportionately high HIV rates among women in sub-Saharan Africa, but male partner resistance and intimate partner violence (IPV) may impact ability to ensure effective use. To support consistent use of the dapivirine vaginal ring (VR), we pilot-tested the impact of the CHARISMA relationship counseling intervention (“CHARISMA”) with women enrolled in the multi-site open-label Microbicide Trials Network (MTN) 025/HOPE trial at the Wits Reproductive Health and HIV Research Institute (Wits RHI) site in Johannesburg, South Africa. Lay counselors used a 42-item tool with five subscales to assess relationships and IPV and provide tailored counseling at enrolment, followed by a booster counselling session at Month 1 and follow-up checks at Months 3 and 6. We evaluated potential impact by examining self-reported ring disclosure to partners, partner clinic attendance, self-reported incident social harms (SH) and IPV, and biomarkers of ring adherence at Wits RHI. We subsequently compared these outcomes at three comparator HOPE study sites using multivariable regression models. Comparator study sites were purposively selected as those most similar to Wits RHI for baseline characteristics identified a priori. At Wits RHI, 95 of 96 (99%) HOPE participants enrolled into the CHARISMA pilot study. Mean age was 30, 36.8% lived with a partner, and 85.3% received their partner’s financial support. During the six months of pilot study follow-up, participants reported: ring use disclosure to partners at 72.7% visits; 4.3% partners attending the research clinic; one partner-related SH; and 9.5% experienced incident IPV. The mean level of dapivirine released from returned used rings was 3.4 mg (SD 1.56), suggesting moderate adherence. Participants in the CHARISMA pilot had high background prevalence and incidence of IPV but were nevertheless able to adhere to ring use, and some male partners came to the research clinic. In adjusted regression models, compared to Wits RHI, partner clinic attendance was lower at all comparator sites; and significantly so at Site A (aRR 0.12, 95% CI 0.00–0.98). Sites B and C had lower levels of dapivirine released (suggesting lower adherence), but this difference was not significant. Site B women were more likely to report ring disclosure to partners at FU visits (aRR 1.12, 95% CI 1.00–1.25). IPV reported during follow-up was significantly lower at Site B (aRR 0.20, 95% CI 0.04–0.98, p = 0.047). CHARISMA taught women skills to decide on levels of ring-use disclosure to partners or others; therefore it is difficult to interpret differences in ring disclosure to partners with other sites. Similarly, CHARISMA heightened participants’ awareness of abuse, possibly increasing IPV reports. Testing CHARISMA under fully-powered controlled conditions will improve understanding of its impact on women’s relationships and ability to use female-initiated HIV prevention methods.
Similar content being viewed by others
References
Kharsany AB, Karim QA. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. The open AIDS journal. 2016;10:34.
Ackermann, L. and G.W.d. Klerk, Social factors that make South African women vulnerable to HIV infection. Health care for women international, 2002. 23(2): p. 163–172.
Dunkle KL, et al. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. The lancet. 2004;363(9419):1415–21.
Jewkes RK, et al. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. The lancet. 2010;376(9734):41–8.
Greig A, et al. Gender and AIDS: time to act. AIDS (London, England). 2008;22(Suppl 2):S35.
Roberts ST, et al. Impact of male partner involvement on women’s adherence to the dapivirine vaginal ring during a phase III HIV prevention trial. AIDS Behav. 2020;24(5):1432–42.
MTN, Monthly vaginal ring advances toward potential approval as new HIV prevention method for women, in Positive opinion by European Medicines Agency paves way for IPM to pursue approvals of dapivirine ring in African countries. 2020: Pittsburgh, PA.
Nel A, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–43.
Baeten JM, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–32.
Agency, E.M., Dapivirine Vaginal Ring 25 mg H-W-2168: Summary of Product Characteristics 2020, [in publication].
Roberts, S.T., et al., Impact of Male Partner Involvement on Women’s Adherence to the Dapivirine Vaginal Ring During a Phase III HIV Prevention Trial. AIDS and Behavior, 2019: p. 1–11.
Palanee-Phillips, T., et al., Impact of partner-related social harms on women's adherence to the dapivirine vaginal ring during a phase III trial. J Acquir Immune Defic Syndr, 2018.
Baeten, J. High adherence and sustained impact on HIV-1 incidence: Final results of an open-label extension trial of the dapivirine vaginal ring. in IAS 2010. 2019. Mexico City, Mexico.
Hartmann M, et al. Generating CHARISMA: development of an intervention to help women build agency and safety in their relationships while using PrEP for HIV Prevention. AIDS Educ Prev. 2019;31(5):433–51.
Wilson EK, et al. Acceptability and feasibility of the CHARISMA counseling intervention to support women’s use of pre-exposure prophylaxis: results of a pilot study. BMC Womens Health. 2021;21(1):126.
Balán IC, et al. Client-centered adherence counseling with adherence measurement feedback to support use of the dapivirine ring in MTN-025 (The HOPE Study). AIDS Behav. 2021;25(2):447–58.
Pallitto C, et al. Testing a counselling intervention in antenatal care for women experiencing partner violence: a study protocol for a randomized controlled trial in Johannesburg, South Africa. BMC Health Serv Res. 2016;16(1):630.
Garcia-Moreno C, et al. Prevalence of intimate partner violence: findings from the WHO multi-country study on women’s health and domestic violence. Lancet. 2006;368(9543):1260–9.
Brown, E., et al., Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV-1 protection, in IAS. 2016: Durban.
Team, T.M.-A.S. A Phase III Trial of the Dapivirine Vaginal Ring for HIV-1 Prevention in Women. in CROI 2016. 2016. Boston, USA.
Sahin-Hodoglugil NN, et al. Degrees of disclosure: a study of women’s covert use of the diaphragm in an HIV prevention trial in sub-Saharan Africa. Soc Sci Med. 2009;69(10):1547–55.
Lanham M, et al. Engaging male partners in women’s microbicide use: evidence from clinical trials and implications for future research and microbicide introduction. J Int AIDS Soc. 2014;17(3 Suppl 2):19159.
Stein ZA. Vaginal microbicides and prevention of HIV infection. Lancet. 1994;343(8893):362–3.
Montgomery ET, et al. The importance of male partner involvement for women’s acceptability and adherence to female-initiated HIV prevention methods in Zimbabwe. AIDS Behav. 2010;15:959–69. https://doi.org/10.1007/s10461-010-9806-9.
MacQueen KM, et al. Social context of adherence in an open-label 1 % tenofovir gel trial: gender dynamics and disclosure in KwaZulu-Natal South Africa. AIDS Behav. 2016;20(11):2682–91.
Montgomery E, van der Straten A, Torjesen K. “Male involvement” in women and children’s HIV prevention: challenges in definition and interpretation. J Acquir Immune Defic Syndr. 2011;57(5):e114–6.
Montgomery ET, et al. An acceptability and safety study of the Duet cervical barrier and gel delivery system in Zimbabwe. J Int AIDS Soc. 2010;13:30.
Montgomery, E., et al., Involving male partners in trials of female-initiated HIV prevention methods in Africa: A review of strategies and evidence, in 5th IAS Conference on HIV Pathogenesis, Treatment & Prevention,. 2009: Cape Town, South Africa.
Montgomery, E.T., et al., Male Partner Influence on Women’s HIV Prevention Trial Participation and Use of Pre-exposure Prophylaxis: the Importance of “Understanding”. AIDS and Behavior, 2014: p. 1–10.
Montgomery CM. The role of partnership dynamics in determining the acceptability of condoms and microbicides. AIDS Care. 2008;20(6):733–40.
Wilson, E., et al., Acceptability and feasibility of CHARISMA: Results of a pilot study addressing relationship dynamics, intimate partner violence and microbicide use. , in HIV Research for Prevention 2018: Madrid, Spain.
Montgomery ET, et al. Social harms in female-initiated HIV prevention method research: state of the evidence. AIDS. 2019;33(14):2237–44.
Funding
The content is solely the responsibility of the authors and does not necessarily represent the official views of the authors’ employers or funders. This program is made possible by the generous assistance from the American people through the U.S. Agency for International Development (USAID) in partnership with PEPFAR through a cooperative agreement (AID-OAA-A-14–00012. The contents do not necessarily reflect the views of USAID or the United States Government. The MTN-025 study was designed and implemented by the Microbicide Trials Network (MTN) funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615 and UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
The study was approved by the Institutional Review Boards at RTI International, all study sites, and was overseen by the regulatory infrastructure of the US National Institutes of Health and the Microbicide Trials Network.
Consent to participate
All study participants provided written informed consent to participate in the study through a consent process approved by local ethical review committees in all research settings.
Consent for publication
All participants were informed that any publication of this study will not use their name or identify them personally.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montgomery, E.T., Roberts, S.T., Reddy, K. et al. Integration of a Relationship-focused Counseling Intervention with Delivery of the Dapivirine Ring for HIV Prevention to Women in Johannesburg: Results of the CHARISMA Pilot Study. AIDS Behav 26, 752–763 (2022). https://doi.org/10.1007/s10461-021-03434-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-021-03434-2