Abstract
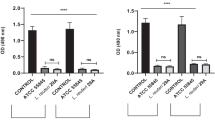
The evolution of antimicrobial-resistant pathogens is a global health and development threat. Nanomedicine is rapidly becoming the main driving force behind ongoing changes in antimicrobial studies. Among nanoparticles, silver (AgNPs) have attracted attention due to their versatile properties. The study aimed to investigate the effects of AgNPs and L-carnitine (LC) on mixed Candida albicans and Staphylococcus aureus in the mice vaginitis model. Study of antimicrobial activity of AgNPs evaluated by Minimum Inhibitory Concentration (MIC) and Minimum Biocidal Concentration (MBC) assays. AgNPs inhibited biofilm formation of microbial strains, which was tested by using crystal violet staining. In the current study, we evaluated the effects of AgNPs and LC in NMRI mice infected intravaginally with C. albicans/ S. aureus for two weeks. The proportion of mice in each stage of the estrous cycle (proestrus, estrus, metestrus, and diestrus) was examined. Histological properties were assessed by hematoxylin/ eosin (H&E) staining of formalin-fixed, paraffin-embedded vaginal tissue sections. Based on the results, MICs of AgNPs against S. aureus, C. albicans, and their combination were 252.3, 124.8, and 501.8 ppm, and their minimum biofilm inhibitory concentration (MBIC) was 500, 250, and 1000 ppm, respectively. The estrous cycle in the treated group was similar to the control. Vaginal histology and cytology showed that LC can improve tissue damages caused by vaginitis and AgNPs. This study demonstrates the promising use of AgNPs as antimicrobial agents and the combination of AgNPs/ LC could be a great future alternative in the control of vaginitis.







Similar content being viewed by others
References
Sayed Ahmed NM (2019) Effect of nursing intervention on knowledge about genital hygienic practices regarding vaginal infection among intrauterine device users and non-users. Int J Nurs Didact 9(1):01–11
Sobel JD, Subramanian C, Foxman B, Fairfax M, Gygax SE (2013) Mixed vaginitis - more than coinfection and with therapeutic implications. Curr Infect Dis Rep 15(2):104–108. https://doi.org/10.1007/s11908-013-0325-5
Noble SM, Gianetti BA, Witchley JN (2017) Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol 15(2):96–108. https://doi.org/10.1038/nrmicro.2016.157
Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MM (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 62:10–24. https://doi.org/10.1099/jmm.0.045054-0
Morales DK, Hogan DA (2010) Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog 6(4):1–4. https://doi.org/10.1371/journal.ppat.1000886
Douglas LJ (2003) Candida biofilms and their role in infection. Trends Microbiol 11(1):30–36. https://doi.org/10.1016/s0966-842x(02)00002-1
Schlecht LM, Peters BM, Krom BP, Freiberg JA, Hänsch GM, Filler SG, Jabra-Rizk MA, Shirtliff ME (2015) Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiol 161(1):168–181. https://doi.org/10.1099/mic.0.083485-0
Kean R, Rajendran R, Haggarty J, Townsend EM, Short B, Burgess KE, Lang S, Millington O, Mackay WG, Williams C, Ramage G (2017) Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00258
Campbell BC, Chan LK, Kim JH (2012) Chemosensitization as a means to augment commercial antifungal agents. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00079
Poovelikunnel T, Gethin G, Humphreys H (2015) Mupirocin resistance: Clinical implications and potential alternatives for the eradication of mrsa. J Antimicrob Chemother 70(10):2681–2692. https://doi.org/10.1093/jac/dkv169
Cui J, Ren B, Tong Y, Dai H, Zhang L (2015) Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 6(4):362–371. https://doi.org/10.1080/21505594.2015.1039885
Navya PN, Daima HK (2016) Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. https://doi.org/10.1186/s40580-016-0064-z
Achiraman S, Archunan G, SankarGanesh D, Rajagopal T, Rengarajan RL, Kokilavani P, Kamalakkannan S, Kannan S (2011) Biochemical analysis of female mice urine with reference to endocrine function: a key tool for estrus detection. Zoolog Sci 28(8):600–605. https://doi.org/10.2108/zsj.28.600
Huang J, Lin L, Sun D, Chen H, Yang D, Li Q (2015) Bio-inspired synthesis of metal nanomaterials and applications. Chem Soc Rev 44(7):6330–6374. https://doi.org/10.1039/C5CS00133A
Singh P, Pandit S, Beshay M, Mokkapati VR, Garnaes J, Olsson ME, Sultan A, Mackevica A, Mateiu RV, Lütken H, Daugaard AE (2018) Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif Cells Nanomed Biotechnol 46(3):886–899. https://doi.org/10.1080/21691401.2018.1518909
Anuj SA, Gajera HP, Hirpara DG, Golakiya BA (2019) Interruption in membrane permeability of drug-resistant Staphylococcus aureus with cationic particles of nano-silver. Eur J Pharm Sci 127:208–216. https://doi.org/10.1016/j.ejps.2018.11.005
Haase A, Tentschert J, Jungnickel H, Graf P, Mantion A, Draude F, Plendl J, Goetz ME, Galla S, Maši A, Thünemann AF (2011) Toxicity of silver nanoparticles in human macrophages: uptake, intracellular distribution and cellular responses. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/304/1/012030
Park S, Lee YK, Jung M, Kim KH, Chung N, Ahn EK, Lim Y, Lee KH (2007) Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol 19(1):59–65. https://doi.org/10.1080/08958370701493282
Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC (2005) In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci 88(2):412–419. https://doi.org/10.1093/toxsci/kfi256
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol Vitr 19:975–983. https://doi.org/10.1016/j.tiv.2005.06.034
Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, Schlager JJ (2006) The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol Sci 92(2):456–463. https://doi.org/10.1093/toxsci/kfl020
Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Kim J, Kim TS (2008) Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal Toxicol 20(6):567–574. https://doi.org/10.1080/08958370701874671
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol 20(6):575–583. https://doi.org/10.1080/08958370701874663
Kart A, Yapar K, Karapehlivan M, Tunca R, Ogun M, Citil M (2006) ‘Effects of L-carnitine on kidney histopathology, plasma and tissue total sialic acid, malondialdehyde and glutathione concentrations in response to gentamicin administration in Balb/C mice. Rev Med Vet 157(4):179–184
Amiri-Moghadam S, Nematy M, Eghtesadi S, Mojarrad M, Jazayeri S, Vosooghinia H, Khosravi A, Salehi M (2015) Effects of L-carnitine supplementation on inflammatory factors and malondialdehyde in patients with nonalcoholic steatohepatitis (NASH). Curr Top Nutraceutical Res 13(3):135–141
Celik F, Kose M, Yilmazer M, Köken GN, Arioz DT, Kanat Pektas M (2017) Plasma L-carnitine levels of obese and non-obese polycystic ovary syndrome patients. J Obstet Gynaecol 37(4):476–479. https://doi.org/10.1080/01443615.2016.1264375
Wayne p (2011) Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing.
Budzyńska A, Sadowska B, Lipowczan G, Maciąg A, Kalemba D, Różalska B (2013) Activity of Selected Essential Oils against Candida spp. Strains. Evaluation of new aspects of their specific pharmacological properties, with special reference to Lemon Balm. Adv Microbiol 3(4):317–325. https://doi.org/10.4236/aim.2013.34045
She P, Liu Y, Wang Y, Tan F, Luo Z, Wu Y (2020) Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida sp. J Appl Microbiol 128(1):88–101. https://doi.org/10.1111/jam.14443
Todd OA, Fidel PL, Harro JM, Hilliard JJ, Tkaczyk C, Sellman BR, Noverr MC, Peters BM (2019) Candida albicans augments staphylococcus aureus virulence by engaging the staphylococcal agr quorum sensing system. MBio. https://doi.org/10.1128/mBio.00910-19
Kalhori Z, Mehranjani MS, Azadbakht M, Shariatzadeh MA (2019) L -Carnitine improves endocrine function and folliculogenesis by reducing inflammation, oxidative stress and apoptosis in mice following induction of polycystic ovary syndrome. Reprod Fertil Dev 31(2):282–293. https://doi.org/10.1016/j.jcyt.2018.09.005
Thein ZM, Samaranayake YH, Samaranayake LP (2007) Dietary sugars, serum and the biocide chlorhexidine digluconate modify the population and structural dynamics of mixed Candida albicans and Escherichia coli biofilms. APMIS 115(11):1241–1251. https://doi.org/10.1111/j.1600-0643.2007.00735.x
Goldman JM, Murr AS, Cooper RL (2007) he rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B 80:84–97. https://doi.org/10.1002/bdrb.20106
Zárate G, Santos V, Nader-Macias ME (2009) Nader-Macias, ‘Protective effect of vaginal Lactobacillus paracasei CRL 1289 against urogenital infection produced by Staphylococcus aureus in a mouse animal model. Infect Dis Obstet Gynecol 2009:48358. https://doi.org/10.1155/2007/48358
Kong EF, Kucharíková S, Van Dijck P, Peters BM, Shirtliff ME, Jabra-Rizk MA (2015) Clinical implications of oral candidiasis: Host tissue damage and disseminated bacterial disease. Infect Immun 83(2):604–613. https://doi.org/10.1128/IAI.02843
Peters BM, Ovchinnikova ES, Krom BP, Schlecht LM, Zhou H, Hoyer LL, Busscher HJ, van der Mei HC, Jabra-Rizk MA, Shirtliff ME (2012) Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiol 158(12):2975–2986. https://doi.org/10.1099/mic.0.062109-0
Alcazar-Fuoli L, Mellado E (2014) Current status of antifungal resistance and its impact on clinical practice. British J Haematol 166(4):471–484. https://doi.org/10.1111/bjh.12896
Murina F, Vicariotto F, Di Francesco S (2016) SilTech: a new approach to treat aerobic vaginitis. Adv Infect Dis 06(3):102–106. https://doi.org/10.4236/aid.2016.63013
Fayaz AM, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan PT, Yao X (2012) Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV- and HSV-transmitted infection. Int J Nanomedicine 7:5007–5018. https://doi.org/10.2147/IJN.S34973
Wady AF, Machado AL, Zucolotto V, Zamperini CA, Berni E, Vergani CE (2012) Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J Appl Microbiol 112(6):1163–1172. https://doi.org/10.1111/j.1365-2672.2012.05293.x
De Groot PW, Kraneveld EA, Yin QY, Dekker HL, Groß U, Crielaard W, de Koster CG, Bader O, Klis FM, Weig M (2008) The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell 7(11):1951–1964. https://doi.org/10.1128/EC.00284-08
Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM (2013) Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine’. Chem Rev 113(7):4708–4754. https://doi.org/10.1021/cr300288v
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275(1):177–182. https://doi.org/10.1016/j.jcis.2004.02.012
Lara HH, Ayala-Núñez NV, Turrent LD, Padilla CR (2010) Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol 26(4):615–621. https://doi.org/10.1007/s11274-009-0211-3
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnol. https://doi.org/10.1088/0957-4484/18/22/225103
EliasS BE (2012) Multi-species biofilms: living with friendly neighbors’. FEMS Microbiol Rev 36(5):990–1004. https://doi.org/10.1111/j.1574-6976.2012.00325.x
Sengupta P, Banerjee R, Nath S, Das S, Banerjee S (2015) Metals and female reproductive toxicity. Hum Experiment Toxicol 34(7):679–697. https://doi.org/10.1177/0960327114559611
Liu F, Mahmood M, Xu Y, Watanabe F, Biris AS, Hansen DK, Inselman A, Casciano D, Patterson TA, Paule MG, Slikker W Jr (2015) Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci. https://doi.org/10.3389/fnins.2015.00115
Agarwal A, Sengupta P, Durairajanayagam D (2018) Role of L-carnitine in female infertility reproductive biology and endocrinology. BioMed. https://doi.org/10.1186/s12958-018-0323-4
Acknowledgements
This paper emanates from the MSc thesis of the first author submitted to the Department of Biology, Faculty of Science, Razi University 67149-67346, Kermanshah, Iran. The authors acknowledge personnel of the Laboratory of Microbiology for technical assistance.
Funding
This study was supported by the intramural fund.
Author information
Authors and Affiliations
Contributions
Dr. Khosrow Chehri and Dr. Mehri Azadbakht designed this experiment. Mozhgan Fatahi Dehpahni did this experiment, analyzed data, and drafted the manuscript. All authors revised the article. Microbiological and histological studies were performed under the supervision of Dr. Khosrow Chehri and Dr. Mehri Azadbakht, respectively.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Approval
In this experimental study, Ethical approval of laboratory animal work was obtained from the Ethics Committee of Razi University, Kermanshah (ethical code: 397–1-03) and mice were kept in the animal room under standard conditions such as 12:12 h light–dark cycle, the beginning of the lighting hour was 8 A.M. and the beginning of darkness was set to 8 P.M., at a constant temperature of 20–22 °C, and free access to food and water.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatahi Dehpahni, M., Chehri, K. & Azadbakht, M. Effect of Silver Nanoparticles and L-Carnitine Supplement on Mixed Vaginitis Caused by Candida albicans/ Staphylococcus aureus in Mouse Models: An Experimental Study. Curr Microbiol 78, 3945–3956 (2021). https://doi.org/10.1007/s00284-021-02652-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02652-0